Fragment Screening
Fragment Based Drug Discovery (FBDD) utilises a range of biophysical techniques, such as surface plasmon resonance (SPR), microscale thermophoresis (MST), differential scanning fluorimetry (DSF) (also termed fluorescent thermal shift assay; FTSA), X-Ray crystallography and nuclear magnetic resonance (NMR), to screen fragment libraries for specific binding to a biological target.
Fragment Based drug Discovery
Sygnature has an in-house proprietary fragment screening collection which can be used to initiate your Fragment Based drug Discovery project. This library can also be supplemented with client or commercial collections if required. The low complexity of the Sygnature Fragment Collection increases the chance of complementarity to any protein surface, such that it may only be necessary to screen several hundred molecular fragments, rather than the hundreds of thousands of compounds often tested during high-throughput screening (HTS) to obtain hits. This fragment-based hit-finding screen is often followed by X-ray crystallography to provide detailed knowledge of exactly how and where the fragments bind to a specific biological target. This structural information then facilitates the optimisation of these low affinity hits – either by careful growing of the fragments, or by combining different fragments together into one molecule.
The small size of the fragments makes subsequent expansion of the hits in an atom efficient manner, through the addition of well-matched functionality, more feasible. This contrasts with the slower, iterative cycles of piece-by-piece optimisation of more complex, larger molecular weight hits (commonly identified by HTS). At Sygnature, this fragment hit optimisation process is particularly effective as many of our fragments have been selected in order to facilitate subsequent medicinal chemistry-driven expansion (so called “poised fragments”).
Surface Plasmon Resonance (SPR) based Screening
Sygnature utilises high-sensitivity Biacore 8K and Biacore T200 SPR instruments that are able to efficiently screen our proprietary fragment library collection at high concentrations. A counter screen against an unrelated control protein can also enable us to identify hits that may be more selective towards the client’s target.

The Biacore 8K is the ‘gold standard’ SPR instrument for fragment-based drug discovery. Its 8 channels and 16 flow surfaces offer the greatest flexibility of set-up, alongside unparalleled throughput and sensitivity:
- Up to 2,300 compounds in a day.
- Full kinetic characterisation of up to 64 fragment hits in only 4 hours.
- Fully-automated compound handling, including robotic and ECHO acoustic dispensing to ensure accuracy down to 2.5 nl.
- Automated, validated analysis packages to rapidly turn around the client’s data.
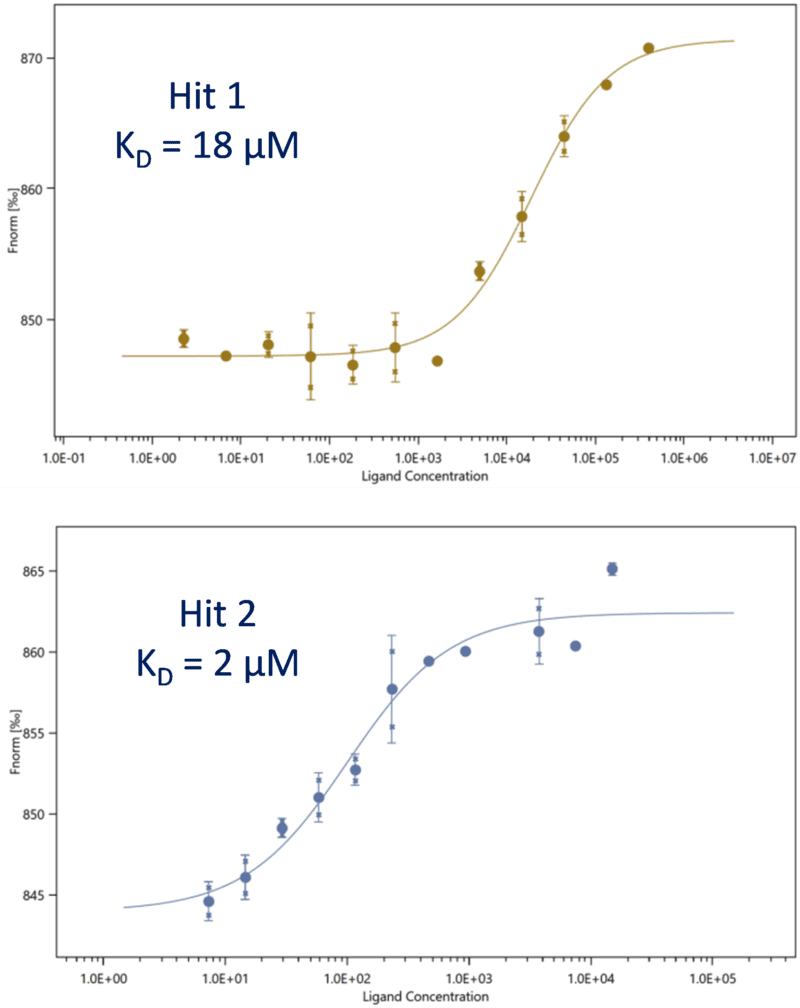
Fragment hits identified via SPR are characterised to accurately measure their affinity (KD). These hits are subsequently confirmed using an array of orthogonal biophysical techniques, which are also available at Sygnature. Upon characterisation, follow-on compounds are synthesised which may also be screened in the various biophysical assays.
MST-based Fragment Screening
Microscale thermophoresis (MST) is a powerful solution-based technology for screening compound libraries and profiling hits for their affinities to a biological target. Its homogeneous solution-based nature makes MST a robust and physiological alternative to SPR, particularly for screening proteins that are not amenable to immobilisation. Sygnature utilises the high throughput automated NanotemperTM Monolith instrument, with a capacity of 4 x 24 capillary chips.
With this capacity it is possible to:
- Screen up to 260 fragments in a day in duplicate.
- Obtain full potency characterisation of up to 24 fragment hits in a day in duplicate.
- Use ECHO acoustic dispensing to ensure accuracy down to 2.5 nl.
- Develop assays with sensitivity sufficient to discern pM to mM affinities.
- Automated, validated analysis packages to rapidly turn around the client’s data.
X-ray Crystallography
Our affiliate company, Peak Proteins, works closely with Sygnature Discovery to provide a commercial fragment screening service. Peak Proteins applies its extensive expertise in protein production and crystallisation to generate appropriate constructs for co-crystallisation, or soak protocols for the biological target of interest in order to screen fragment libraries or determine binding mode of hits using X-ray crystallography. Constant review of binding interactions observed in such X-ray structures enables ligand efficient optimisation to provide potent and selective leads for advancement towards development.
NMR-based Fragment Screening
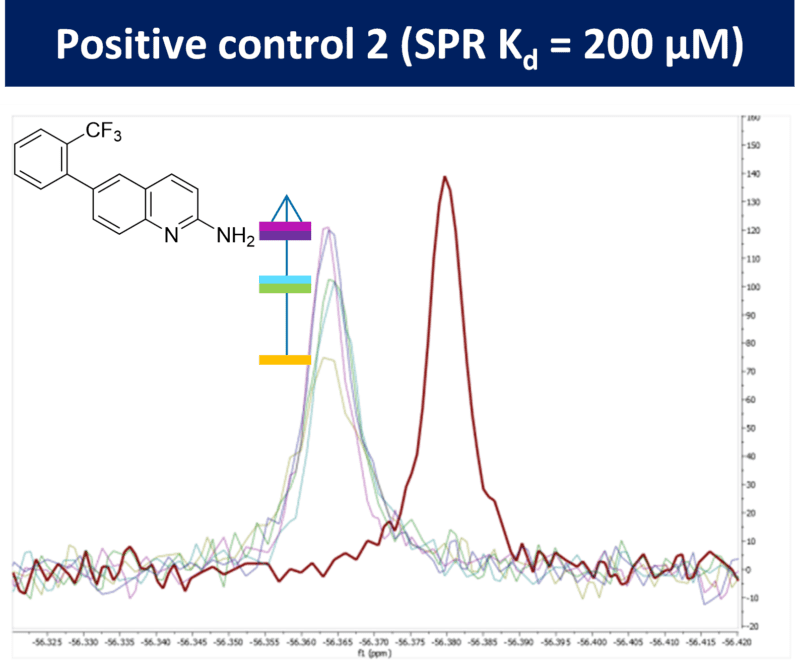
As a solution-based and label-free technology which allows the direct observation of binding events, NMR is well suited for fragment screening. Sygnature utilises several cryoprobe-equipped Bruker 500 to 800 MHz NMR spectrometers, which offer excellent resolution and throughput. These instruments also have auto-samplers for high-throughput screening capabilities, facilitating screening of the fragment library in a matter of hours. Sygnature Discovery has experience with fragment-based NMR screening methods using this instrumentation, including saturation transfer difference (STD) and waterLOGSY.
Sygnature also has experience with 19F-NMR techniques for fragment screening, both for direct binding of fluorine-containing fragments and in screening in competition with non-fluorine-containing fragments. 19F-NMR has the added advantage of increased throughput with the ability to screen mixtures of samples without spectral overlap. Sygnature can apply any of these approaches to either a fragment library screen or a hit profiling study.