Target Validation
Many drugs in development face failure in clinical trials due to issues like lack of efficacy and toxicity. These problems can often be traced back to inadequate pre-clinical target validation techniques.
Target validation is the critical first step in the discovery of a new drug, and it typically takes 2-6 months to complete. This essential process involves applying a range of techniques to demonstrate that the drug’s effects on the target can offer therapeutic benefits within an acceptable safety window. Early and comprehensive target validation helps establish a clear connection between target manipulation and disease efficacy, significantly increasing the chances of success in clinical trials. Once a target achieves an acceptable level of validation and disease linkage, the project advances to the hit identification phase.
Effective Target Validation Techniques
The spectrum of target validation techniques are summarised below:
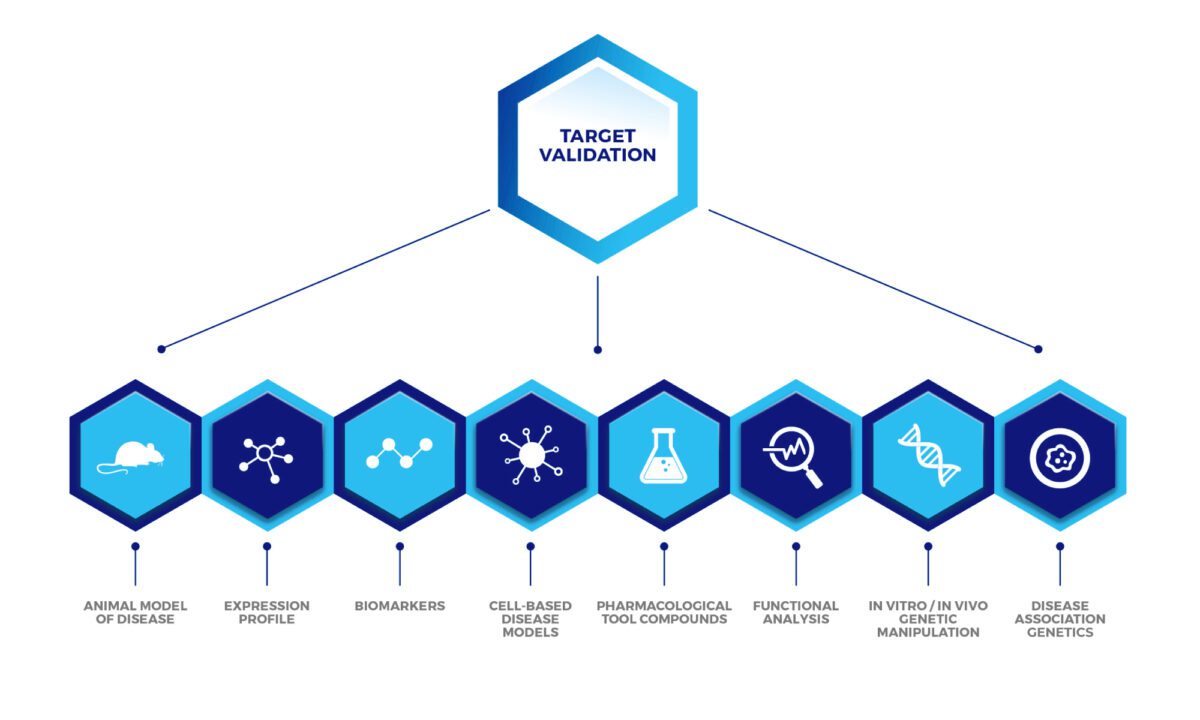
At Sygnature Discovery, we have a proven track record of validating disease-modifying targets across various target classes and therapeutic areas. Our expertise lies in tailored assay development, offering a complementary suite of techniques to build strong evidence for your biological targets. Our target validation services include:
Functional analysis
- Value-adding in vitro assays to measure the biological activity of the target, characterise pharmacology and assess the effects of modulating function.
- Use of ‘tool’ molecules to demonstrate desired in vitro biological effect.
Expression profile
- mRNA and protein distribution pattern to determine target expression and function in both healthy and disease states.
- Correlation of expression with disease progression or exacerbation using disease-relevant cells/tissue.
Cell-based models
- 3-dimensional (3D) cultures and co-culture models, human stem cells (iPSC) , including access to disease cells.
Biomarkers
- Biomarker identification and validation using multiple techniques to include transcriptomics (qPCR platforms), protein analyte detection (Luminex) and flow cytometry.
