Antibody Drug Conjugates
Introducing a novel payload-focused approach to next generation ADC development
NewPath ADC offers integrated solutions for antibody drug conjugates, designed by highly skilled multidisciplinary teams covering medicinal chemistry and protein sciences, as well as in vitro and in vivo assessment (including DMPK).

While other providers focus on a conventional, well-trodden development pathway, Sygnature Discovery’s ADC solutions prioritize payload optimization to enhance a drug’s efficacy and selectivity, thereby maximizing its therapeutic benefit. This translates into a better-performing end-product, enabling your success.
Big ADC problem? Small molecule solutions
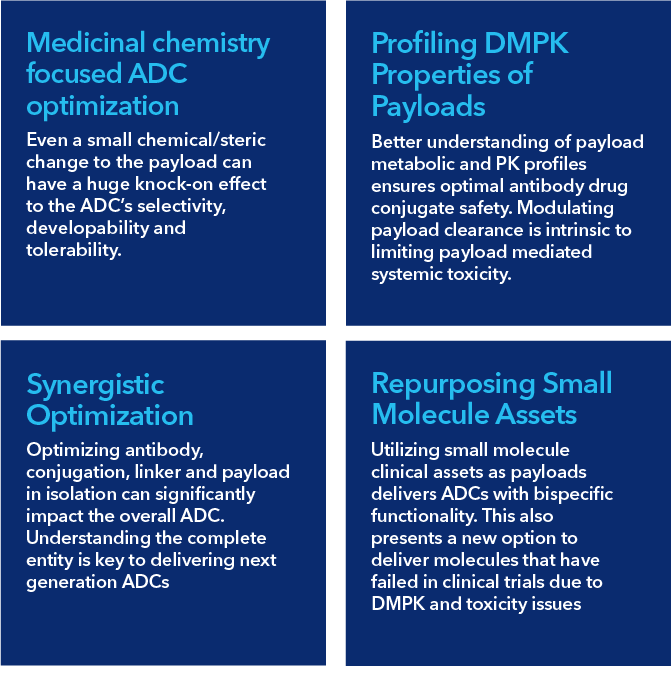
Our strategy to deliver therapeutically relevant ADCs is based on our expertise in four key areas:
Holistic development of novel antibody drug conjugates
Our comprehensive antibody drug conjugate framework integrates in vitro and in vivo profiling to optimize potency, efficacy, and safety. It also addresses key challenges from early-stage design to IND-enabling studies, while focusing on developability, safety profiling, and clinical progression. This ultimately leads to better patient outcomes.
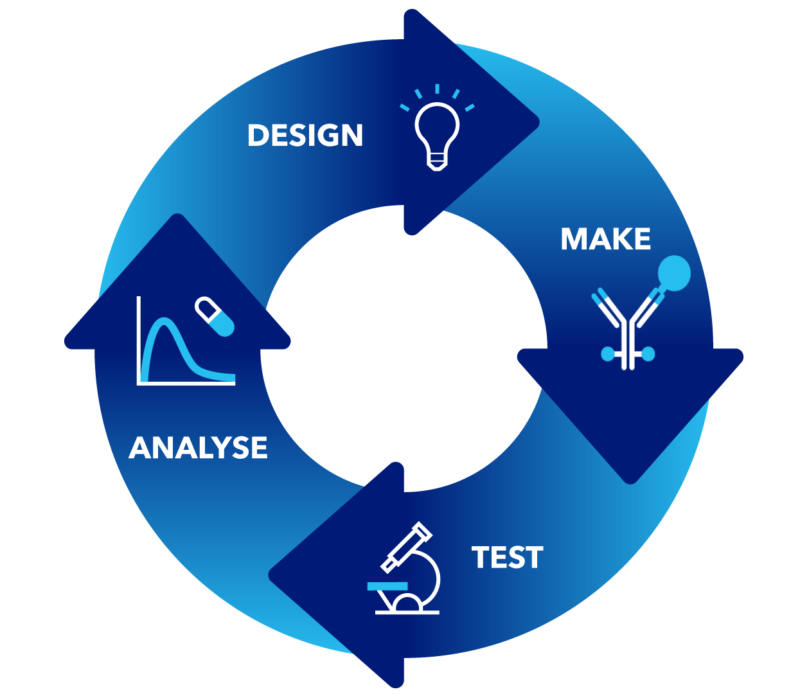
Design, Make, Test, Analyze
Whilst not traditionally considered during antibody development (and, by extension, ADC development), we bring small molecule thinking to large molecule discovery.
During an integrated antibody drug conjugate development project, our chemistry expertise informs the optimization of key components (such as the antibody, conjugation techniques, linker, and payload design), while our protein science and biology capabilities ensure a streamlined path from designing your antibody drug conjugate to testing it.
Our ADC development design & make capabilities:
- Antibody expression and purification
- Linker payload synthesis
- Conjugation
- QC/Characterisation
Our ADC development test & analyze capabilities:
- Biophysical Screening
- In-vitro PD analysis
- In-vitro DMPK analysis
- In-vivo Analysis
- Developability
“Shifting the ADC focus from antibody to payload”
 Josh Greally, ADC Lead at Sygnature Discovery, discusses why he is advocating for disruption in the market with a payload-first approach to ADC development in Drug Target Review’s latest article titled ‘Shifting the ADC focus from antibody to payload‘.
Josh Greally, ADC Lead at Sygnature Discovery, discusses why he is advocating for disruption in the market with a payload-first approach to ADC development in Drug Target Review’s latest article titled ‘Shifting the ADC focus from antibody to payload‘.
Discover how Sygnature’s NewPath ADC platform is applying this approach, and why a payload-first strategy could unlock new levels of therapeutic potential.
Why choose Sygnature Discovery?
Since 2011, Sygnature Discovery has delivered 56 novel pre-clinical and 34 clinical compounds, with its scientists named on over 225 patent applications. Therapeutic areas of expertise include oncology, inflammation and immunology, neuroscience, metabolic diseases, infectious diseases, fibrotic diseases, and more.
We believe our small molecule focus offers an advantage when it comes to ADCs. After all, it’s the linker and payload that differentiates an ADC from an antibody, and optimizing these are our core areas of expertise. Whilst we have the capabilities (and significant experience) to produce antibodies, conjugate, analyze, and screen for developability, it is our extensive capabilities in the design, synthesis and optimization aspects of both the linker and the payload that really sets us apart. In the ADC field, payload development is still in its infancy, but our customers are already leveraging our platform to help bring the next wave of more selective and safer ADCs toward the clinic.
Your FAQs: Antibody Drug Conjugates
Q1: How does Sygnature Discovery’s track record in ADC development support benefit customers?
A1: Sygnature’s ADC platform provides an experienced team with access to multiple disciplines who can initiate/evolve ADC drug discovery campaigns. We specialize in payload-focused ADC development. We believe that the next generation ADCs will feature more specifically targeted therapeutics as payloads and we are already actively involved in the delivery of ADCs in this space.
Sygnature has extensive capabilities for the development of the next generation of payloads. We have expertise in multiple MOAs and can optimize your existing payloads to improve both therapeutic window and ADC biophysical properties. We are a highly experienced team that can develop all types of assay (biophysical, in– vitro and in– vivo assessment of both PD and PK attributes), tailored to ADC projects.
Q2: What if I have a drug with poor PK and can’t get a therapeutic exposure..?
A2: By conjugating to an ADC, delivery to tumour is enhanced vs systemic exposure, increasing local drug concentration. And with variable DARs, we can help control that exposure to help control ADC PK and properties. We can use our experience in the area to examine how small changes in linker and payload chemistry, DAR and other factors can have dramatic impacts on both PK and efficacy.
Q3: What if my targeted therapy (small molecule or biologic) has systemic on-target toxicity so efficacy is limited by side effects...?
A3: We can use a carefully designed ADC to deliver your compound direct to the site of action, alleviating this off-target toxicity. And deploying our medicinal chemistry expertise, we can investigate how we best tailor the stability of the compound whilst maintaining potency, so any compound that enters the systemic circulation after lysosomal clearance and cell death is rapidly eliminated.
Q4: How can Sygnature Discovery help solve my ADC development challenges…?
A3: We are small molecule focused, however we do support (and have a strong track record in) other modalities, including biologics, ADCs and peptides. We have a large protein science department with extensive experience in antibody/biologic expression, purification, QC and developability assessments.