Biophysical Technologies
At Sygnature Discovery, we utilise various biophysical technologies to create assays for diverse targets, integral to every stage of the drug discovery process, from hit identification to candidate selection. Explore this page to learn about the technologies we use and their key benefits.
Our skilled protein science team can provide in-house production of proteins to support all these assay formats if needed. With a strong track record, this experienced team excels in producing a diverse array of proteins, offering various tagging options customisable to specific applications.
Surface plasmon resonance (SPR)
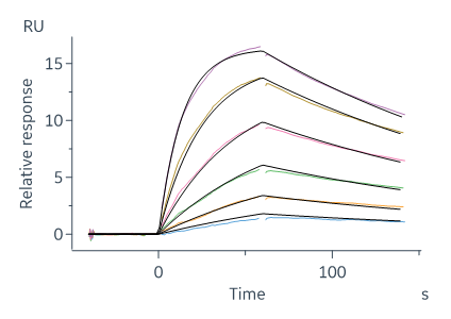
Surface plasmon resonance (SPR) is a key biophysical technology at Sygnature Discovery, renowned for its widespread industry use. It enables the analysis of affinity, kinetics, and stoichiometry across various target classes and potencies.
At Sygnature, we use top-of-the-line BiacoreTM instruments from Cytiva, ensuring high throughput and superior data quality for every project. Our in-house SPR equipment comprises:
- 2x BiacoreTM 8K+
- 3x BiacoreTM 8K
- 1x BiacoreTM T200
This covers mM binders, such as fragments, through to sub pM interactions (such as late stage small molecules or very high affinity biological interactions, Tollenaere et al 2023 https://doi.org/10.1016/j.xjidi.2023.100214).
At Sygnature Discovery, we excel in various SPR assay formats, covering a broad spectrum of programs. Our unique expertise includes evaluating covalent binders via SPR, which excels in sensitive, time-sensitive assay detection. Our strength lies in creating detailed, highly sensitive SPR assays that provide comprehensive kinetic and stoichiometric data with high throughput.
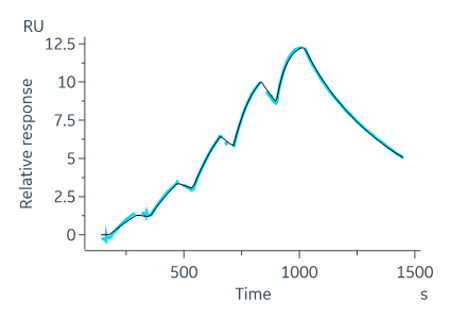
Microscale Thermophoresis (MST)
Microscale Thermophoresis (MST) is a technology that uses fluorescently labelled proteins to analyse small molecule binding in solution.
MST primarily measures molecules’ movement in temperature gradients, incorporating two effects: thermophoresis (movement towards or away from heat) and Temperature-Related Intensity Change (TRIC), where temperature shifts affect the fluorophore’s brightness. These effects, influenced by changes in charge, hydration shell, or molecular mass, are monitored at varying concentrations to determine molecular interactions and affinity data in MST assays.
MST measures molecular motion in temperature gradients, combining thermophoresis (movement toward/away from heat) and Temperature-Related Intensity Change (TRIC), where temperature shifts affect fluorophore brightness. These phenomena, sensitive to changes in charge, hydration shell, or molecular mass, alter the observed signal. Monitoring these changes at various concentrations forms the basis of MST assays for determining molecular affinity data.
MST, compared to SPR, offers less data but excels in its setup, eliminating the need for surface capture and keeping samples in solution. This is advantageous for sensitive proteins and multi-component complexes with short residence times, often undetectable by SPR. MST is less restricted by molecular weight and buffer composition in small molecule assays and can analyse interactions with various molecules, including RNA/DNA, lipids, carbohydrates, and proteins, even membrane-bound ones.
This technology measures affinities from mM to nM. At Sygnature, we use the high-sensitivity Monolith NT.Auto (PicoRed) for a range of targets, similar to SPR capabilities. MST is notably effective for DNA binding proteins, where it outperforms flow-based systems in multi-component assay setups.
Nuclear Magnetic Resonance (NMR)
Nuclear Magnetic Resonance (NMR) is a key technique for chemists and structural biologists. Our in-house team of NMR experts specialises in ligand binding experiments using proton or fluorine NMR, particularly effective for small molecule-biomolecule binding assays and fragment screening, where it excels in detecting mM affinity binders. Additionally, we extend our NMR capabilities through partnerships with the University of Birmingham and the University of Leeds, national centres for NMR spectroscopy. This collaboration provides access to a broader range of equipment and additional expertise.
Sygnature’s protein science team provides protein-observed NMR services, enabling the determination of binding site locations and structural analysis, among other techniques. We have in-house 19F NMR capabilities and, through our partners, access to a broader array of probes and field strengths.
| In-house: |
|
| Partner access: |
|
Mass Spectrometry (MS):
Mass spectrometry (MS) is a highly sensitive and specific method used by Sygnature Discovery for detecting and simultaneously monitoring multiple molecules in a sample. Our team, with extensive MS expertise and diverse instrumentation, applies MS in Biophysics for various assays, including enzyme activity, label-free receptor binding, and identifying new protein ligands. We measure enzyme activity through MS by tracking product formation and substrate consumption and developing custom assays to determine parameters like kcat/Km, IC50 values for enzyme inhibitors, and inhibition mechanisms.
Our MS-based enzyme assays utilize natural substrates, avoiding coupled reactions. Additionally, we offer MS-based techniques for quantifying ligand binding to membrane receptors, complementing our radioligand binding services. This label-free method is crucial for receptor target drug discovery, providing key data on binding confirmation, affinity, and kinetics.
Intact protein MS is a powerful technique for the detection of covalent binders. Covalent drugs may have advantages over non-covalent molecules, such as greater potency and duration of action. Deconvolution of intact mass/charge spectra acquired using high-resolution MS enables our team to discover new starting points or investigate compounds’ modes of action.
Our MS team is backed up by a wide pool of experience from across the company, including from the DMPK, Protein Science and Separation Science departments, which allows us to draw on expertise from a variety of applications to develop the right assay for the target.
Thermal shift assays (TSA)
Sygnature Discovery utilizes Mass Spectrometry (MS) for sensitive detection and monitoring of multiple molecules, specialising in enzyme activity assays, label-free receptor binding, and protein-ligand identification. Our team develops custom MS-based enzyme assays for key parameters like kcat/Km and IC50 values, using natural substrates. Additionally, we offer MS techniques for quantifying ligand-receptor binding in drug discovery, providing essential data on binding affinity and kinetics without the need for labelling.
Thermal shift assays (TSA) at Sygnature Discovery are primarily used for straightforward target engagement determination, offering a robust and often successful approach even for challenging targets. If a protein’s melt curve can be established, TSA is likely applicable. We have advanced this technique for some targets by estimating KD values using both titration methods and analysing slope and Tm data from a single concentration, although this approach may not suit all assay systems.
Additional Technologies
Sygnature Discovery enhances its in-house technologies by accessing additional methods like ITC and DLS through partnerships, often operated by our scientists. We continuously seek to expand our technological capabilities to meet diverse project needs.
