Cleaning out disease – PROTACs on the rise
There has been a lot of interest recently in PROTACs, or proteolysis-targeting chimeras.
In this novel type of potential drug treatment, the PROTACs hijack the cells’ protein destruction mechanism and redirect it to destroy a protein that causes disease, rather than interfering with the function of the target protein. Sygnature Discovery’s Dr Roland Hjerpe delves further into this topic.
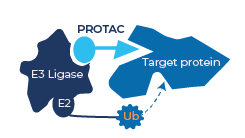 So how do PROTACs carry out this work? Well, they are small molecules that exploit the ubiquitin–proteasome system within the cell that acts as a ‘garbage disposal’ mechanism. Ubiquitin ligase enzymes tag proteins that are destined for destruction with the small protein ubiquitin. This tag is recognised by the proteasome, a large, macromolecular protease enzyme whose function is protein destruction. Although ubiquitin-tagging can have other effects too, it is this degradative function that the PROTAC technology is designed to exploit.
So how do PROTACs carry out this work? Well, they are small molecules that exploit the ubiquitin–proteasome system within the cell that acts as a ‘garbage disposal’ mechanism. Ubiquitin ligase enzymes tag proteins that are destined for destruction with the small protein ubiquitin. This tag is recognised by the proteasome, a large, macromolecular protease enzyme whose function is protein destruction. Although ubiquitin-tagging can have other effects too, it is this degradative function that the PROTAC technology is designed to exploit.
The PROTACs created by drug designers are bi-functional molecules, where one end binds to a ubiquitin ligase enzyme, and the other to the disease-associated protein they are looking to destroy. This, effectively, allows the protein to be ubiquitin-tagged for destruction by the proteasome.
This mechanism has several potential advantages over more traditional mechanisms of drug activity. It can be difficult, if not impossible, in some cases to design a small molecule that binds specifically to an active site. As PROTACs do not directly inhibit a function, they do not have to bind to active sites in the target protein –they only need to bring the target and the ubiquitin ligase together. It doesn’t matter whether they bind to the active site, or somewhere else on the target protein. This opens up the possibility of targeting proteins previously considered to be undruggable.
 Another advantage of PROTACs is that they can function sub-stoichiometrically. Classical drugs need to stay bound to the target to have an effect, but once a PROTAC has mediated the degradation of a protein, it can bind to another target protein and start the cycle again. For this reason, one PROTAC molecule can drive the destruction of several target protein molecules, which could lower the required dose of a drug, and might also limit potential side-effects.
Another advantage of PROTACs is that they can function sub-stoichiometrically. Classical drugs need to stay bound to the target to have an effect, but once a PROTAC has mediated the degradation of a protein, it can bind to another target protein and start the cycle again. For this reason, one PROTAC molecule can drive the destruction of several target protein molecules, which could lower the required dose of a drug, and might also limit potential side-effects.
Hundreds of ubiquitin ligases are known, but only a handful have thus far been used to generate PROTACs. As such, there is a huge amount of untapped potential, not to mention plenty of IP space, for development of PROTACs that exploit new ubiquitin ligases. These can have properties that will be highly beneficial for drug development, potentially opening up for PROTACs that exert their effect only in a specific tissue of choice, or that are restricted to a specific subcellular compartment. Although exciting in many ways, there are challenges, such as how to best find small molecules that bind to a new ubiquitin ligase, how to link these to the small molecule that binds the target, and finally how to design the bifunctional PROTAC molecule in such a way that it spatially orients the ubiquitin ligase and the target in an arrangement that allows the ubiquitin tagging to take place efficiently. Moreover, redirecting a ubiquitin-ligase away from its natural target may have unforeseen consequences, potentially leading to deregulation and accumulation of the natural target, which may have effects that could be difficult to predict. Undoubtedly, the coming years will provide novel insights into this, and other questions, as more and more PROTAC modalities are explored.
PROTACs have potential in multiple disease areas where protein degradation might prove beneficial. Cancer and inflammatory disease both represent important areas of unmet medical need, and if the PROTACs proposition proves successful, they could have a real impact for patients.
There are many other fascinating treatment ideas that could be explored. For example, might PROTACs be effective in treating neurodegenerative diseases? Many of these are thought to involve the accumulation of misfolded proteins. What if PROTACs could remove them? This is not without its challenges – the PROTAC molecule would need to cross the blood–brain barrier, a tall order given that PROTACs generally have inherent properties that would seem to exclude them from the brain. Having said this, PROTACs have recently been claimed to achieve brain penetration in pre-clinical studies, opening up an avenue for targeting various neurodegenerative conditions.
Although it is still early days for PROTAC technology, Sygnature is well placed to help clients develop new PROTAC molecules. Along with expertise in the ubiquitin system, and the wider field of protein degradation, we are well versed in the different assays required for PROTAC development, in particular with respect to Surface Plasmon Resonance, a technique that can be utilized to examine PROTAC ternary complexes. This, as well as our computational and medicinal chemistry capabilities, our hit-finding expertise (HTS & fragment-based screening) and broad expertise in various disease areas, puts us in a good position for PROTAC development.
At the time of writing, there are two candidate PROTAC drugs in clinical trials, adding to the buzz around the area. ARV-110 and ARV-471 from Arvinas are orally available PROTACs that target the androgen receptor (AR) and the estrogen receptor (ER) and are designed to treat metastatic castration-resistant prostate cancer and locally advanced or metastatic AR-positive breast cancer, respectively. If these show success, much more interest in developing PROTACs for other diseases can be expected.
We continually engage with our industry on a range of drug discovery topics. If you would like to discuss our bioscience capabilities or drug discovery in general then we’d love to hear from you. You can get in touch by using any of the contact forms.
