Why DMPK is the glue of R&D
DMPK influence starts at the very beginning and transcends into the clinic and beyond, arguably playing the most pivotal role in the drug discovery process for many projects. Dr Richard Weaver, SVP – Preclinical Development, explores both the common pitfalls and the best approaches to ensure success in your DMPK strategy.

A pharmacologically active research compound or drug must engage its target in the body at the optimal concentration and desired duration to elicit its wanted effect.
 The maximum in vivo exposure is defined by two things, namely the dose administered and the inherent chemical structure of the molecule. How the body deals with the compound in terms of ADME properties is completely “locked in” by the chemical structure. Therefore, when engaging with DMPK scientists, the chemical structure should be the defining starting point of the DMPK conversation and strategy.
The maximum in vivo exposure is defined by two things, namely the dose administered and the inherent chemical structure of the molecule. How the body deals with the compound in terms of ADME properties is completely “locked in” by the chemical structure. Therefore, when engaging with DMPK scientists, the chemical structure should be the defining starting point of the DMPK conversation and strategy.
Most drug discovery scientists will know they need to get DMPK data, but many don’t know what data to get, which data is pivotal in improving properties, or even what to do with the data. Very simply, DMPK is the glue that links wanted (and unwanted) pharmacological effect and unbound exposure.
Therefore, DMPK transcends the design within medicinal chemistry, pharmacology, safety and pharmaceutical development. A compound with low potency and poor DMPK properties will be, by definition, not viable. At best, the dose will be extremely high, with an unacceptable dosing frequency and poor safety profile.
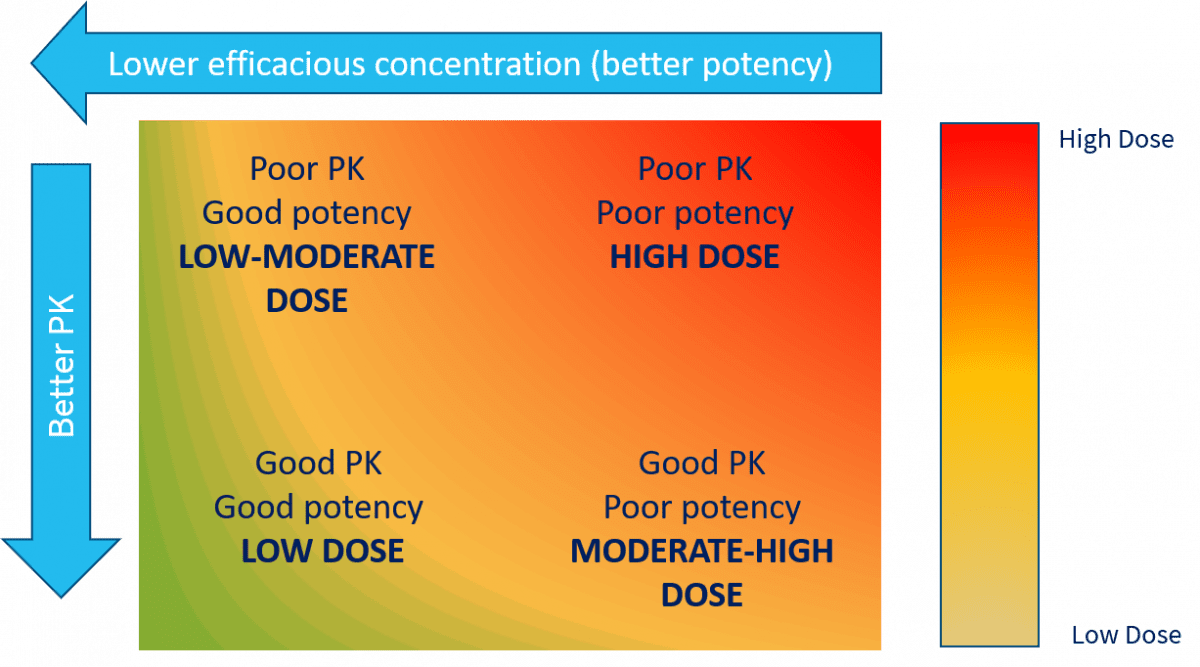
Assessing where you are
Every single research compound and drug is somewhere in the rectangle in Figure 1:
The key, in terms of improvement, is first understanding where your compound is on the spectrum of this rectangle. Assuming an oral drug, the minimum data required to “pin the tail on the donkey” is a relevant measure of in vitro potency that translates relevantly through to in vivo potency and some key DMPK measurements.
Typically, these would be relevant metabolic stability, plasma protein binding, a measure of passive permeability and ideally some pre-clinical IV and PO pharmacokinetics. With these measurements, it’s possible to estimate both the likely human PK parameters and efficacious dose.
 The aim in drug discovery is to move towards the bottom left of Figure 1. But how do you do that if the DMPK support you have outsourced is just a “capability service”? The answer is you can’t. DMPK must be an integral and often the key influential area in drug discovery.
The aim in drug discovery is to move towards the bottom left of Figure 1. But how do you do that if the DMPK support you have outsourced is just a “capability service”? The answer is you can’t. DMPK must be an integral and often the key influential area in drug discovery.
Having worked in the industry for 24 years, I’m yet to find a medicinal chemistry team who can’t make potent compounds in a project. But a potent compound is not a drug. I’ve lost count of the number of times that the project team has not managed to get the sufficient balance of potency and DMPK properties to make it.
Sometimes it’s simply impossible, as the pharmacophore is intrinsically linked to the poor DMPK properties and any attempts with isosteres have failed. The point is to recognise where you are and if a compound series can be optimised, however trivial this may seem, or if the programme should actually be stopped.
“Show me the structure”
Five key questions I ask my colleagues or clients every single time when I first get involved:
- Can you share the chemical structure?
- Does your molecule contain an ionisable functional group or groups?
- What is its kinetic solubility?
- What is the measured logD4?
- What is the molecular weight?
Answers to these 5 questions will tell me what the issues are going to be (there are always some, often multiple), how difficult these may be to resolve, and help to define the DMPK screening strategy.
ON-DEMAND WEBINAR
How DMPK should integrate with and influence projects in discovery and beyondWith Dr Richard Weaver |
A one-size DMPK screening strategy is not appropriate
 As the infinitely variable ADME properties are fixed by the chemical structure, why should a single DMPK screening strategy work for all compounds?
As the infinitely variable ADME properties are fixed by the chemical structure, why should a single DMPK screening strategy work for all compounds?
For example, for a low logD7.4 carboxylic acid with low passive permeability, screening for metabolic stability in microsomes or hepatocytes will not likely be fruitful. Compounds of this nature are often moved around and out of the body by active transport. Hepatic and renal uptake, followed by metabolism in the former, will be the likely dominant clearance pathways.
A regular microsome or hepatocyte assay will not be able to predict this. What will be needed in this scenario is an active uptake assay in hepatocytes and some measure of passive and active renal clearance. The latter can be measured from IV PK studies in appropriate species.
Free-drug exposure – a concept many people still don’t understand
Only unbound drug can contribute to wanted and unwanted pharmacology, and also only unbound drug can be metabolised and excreted. Therefore, to allow the conversion from total blood or plasma exposure in vivo, one has to know what the plasma protein binding is.
Note that one only needs to know what it is. Plasma protein binding cannot be optimised. This is a story for another article. The take-home message for now is that it should not be a target for “improvement” because it cannot be improved. Likewise, it should not be front-loaded as a decision-making assay in a screening cascade and should only be back-filled for compounds that progress to in vivo PK studies for in vitro-in vivo extrapolation (IVIVE).
It is often prudent to measure human plasma protein binding at the same time to check for any differences between species, as a safety margin could easily be eroded to nothing if there are differences in the wrong direction.
How should DMPK fit into Drug Discovery, Drug Development and beyond?
Quite simply, DMPK thinking should be implemented from the outset, when the first “hit” or competitor-busting compounds are identified. And it is essential that the DMPK team contains individuals with chemistry knowledge and thinking.

It is arguably the most influential discipline, as how many projects have you worked on where DMPK has been an issue? I would say all projects. And that’s before we consider how DMPK scientists can help predict a future likely safety margin, drug-drug interaction (DDI) potential, special population DMPK considerations, informing the formulation teams of whether they will be able to fix poor bioavailability, and helping refine the dose and PK prediction as Phase 1 and Phase 2 data becomes available.
DMPK influence starts at the very beginning and transcends into the clinic and beyond, well after medicinal chemistry has stopped. The best DMPK scientists are constantly thinking “could this be a drug?” and not just blindly helping to support a client get to candidate drug nomination.
Why would you not give this the attention it deserves? Getting a successful drug to patients depends on the best multi-discipline integrated R&D thinking and influence from day one to the market and beyond.
We continually engage with our industry on a range of topics. If you would like to discuss drug discovery, our capabilities or what we are about, then we’d love to hear from you. You can get in touch by using any of the contact forms on our website.