Effective drug design: insights from DMPK
Modelling and simulation approaches developed under the DMPK umbrella utilising animal in vivo data and advances in in vitro technologies continue to raise expectations for drug design with respect to prediction of clearance and efficacy in the clinic (and where appropriate potential for DDIs). These innovations have raised the profile of DMPK scientists and ensured the DMPK skillset is confirmed as a critical component of integrated drug discovery programmes.
To move forward with confidence on successful outcomes in the clinic, data obtained from pre-clinical pharmacokinetic and pharmacodynamic studies need to be integrated to make rational decisions leading up to and at candidate selection. To achieve this, we need:
- A prediction of human PK
- A measure of efficacy that translates to humans
- A strategy that allows early judgements on the success of potential treatments (biomarkers)
- A strategy in place to manage potential DDIs
Mechanism based PK/PD models fall within the domain of DMPK scientists who can use the models to integrate the above into data-based, science driven predictions that demonstrate understanding of the processes involved thereby allowing different scenarios to be mapped out.
However, the journey to get there is not necessarily straight forward. Most drug discovery projects will be aware that they need DMPK data. But which data to generate and when is it pivotal in improving the overall properties of a chemical series?
Each compound falls somewhere into the matrix below:
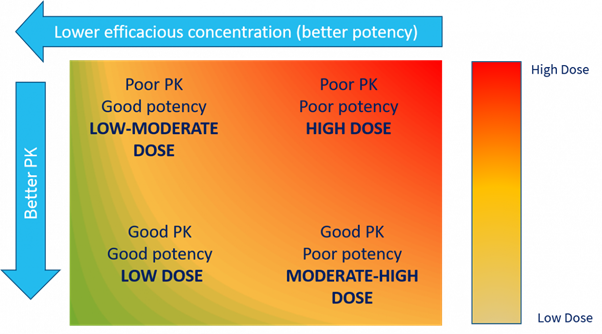
Selecting the right DMPK data to drive successful drug design
Determining the acceptable balance of potency and PK is evaluated as part of the DMPK investigative strategy. This is effectively the glue which links unbound concentration and pharmacological effect, be that a wanted or unwanted effect in the case of toxicity.
A compound with low potency and poor DMPK properties will not be a viable drug. The dose will be extremely high, with an unacceptable dosing frequency and poor safety profile. Chasing high potency may not give the result required, the same goes for chasing good PK. Where the DMPK expert can help, is to calculate what is an acceptable exposure that will drive the required therapeutic effect.
Assuming a drug is to be delivered via the oral route drivers of exposure such as metabolic stability, plasma protein binding and permeability can be investigated and correlated with comparative iv and oral PK studies. However, other factors may also come into play, such as CYP effects, transporters, enterohepatic recirculation etc. A DMPK expert will be able to analyse the data and point to areas required for further investigation to better understand how the body is treating the drug and what areas warrant further clarification to allow optimisation of the human dose.
The maximum in vivo exposure of a compound is defined by two things:
- The dose administered
- The inherent chemical structure of the molecule
How the body deals with the compound is defined by the structure of the compound, thus the chemical structure should be the starting point of the DMPK discussion and screening strategy to establish structural activity relationships that can be used to inform on chemical design and see the potential to ‘overlap’ with the SAR of the other parameters required for efficacy.
Understanding the best DMPK strategy for each drug discovery stage
DMPK spans drug discovery, post hit-ID through into the clinic and beyond working initially with hundreds and maybe thousands of compounds to select a single candidate drug for preclinical development. Therefore, different objectives are employed for the DMPK strategy at different stages of drug discovery process.
In the early stages of hit-to-lead and lead exploration the focus is on early assessment and direction setting. Working with exemplars of multiple chemical series using the DMPK toolbox and a tiered approach working through:
- In silico predictive models
- Phys chem – solubility, logP, pKa (acid/base), log D
- In vitro DMPK assays (e.g. Metabolic stability, PPB, permeability)
- CYP inhibition and induction
- In vivo benchmarking
- IVIVE potential
- Oral bioavailability
…to identify ‘issues’ and the assays required to investigate or establish SAR – and if necessary develop ‘bespoke’ assays with appropriate validation that can be ready and available for use during lead optimisation. In this initial stage DMPK assays help to rank these early-stage compounds and prioritises those chemical entities that are to be selected for further development. Just as importantly it identifies those which are not destined to be drugs and should be dropped with no further resources allocated.
In early lead optimisation the move is towards generation of SAR to allow ‘multiparameter optimisation’ identification of the chemical space where our candidate drug will come from. In DMPK the aim is to be able to use in vitro assays and in vitro to in vivo extrapolation to reduce cycle times. As well as to inform on chemical design and only using in vivo data for selected compounds for confirmation. It is also a time when we can start to get an indication of concentration–effect relationships and therefore can also carry out early human predictions of dose. A watching brief on the other DMPK parameters e.g. potential for DDI, TDI etc. are also monitored as the chemical series progresses.
Late lead optimisation now focuses on narrowing down to compound selection of our potential candidate. Oral bioavailability optimisation via formulation modifications can be employed as dose proportionality is investigated together with higher species PK, the development of PK/PD models to allow refined human predictions.
Strategic input and targeted investigation by DMPK are key to successful drug design
Once a potential drug candidate is identified, it is all about compound characterisation and obtaining a definitive prediction of the human efficacious dose. The studies also contribute to risk identification (i.e. indicating what liabilities are potentially being carried into preclinical development) and the preparation of mitigation plans indicating the critical studies that will be decision making in the preclinical development phase. These include:
- Human PK and DDI predictions utilising allometry and/or PBPK-IVIVE
- Human dose predictions (PK/PD) advising on special population based DMPK considerations
- Cross species metabolism studies to indicate relevant tox species
- Early repeat dose studies to identify potential dose effects, time effects and sex differences in PK and metabolism.
In the discovery of new drugs it’s clear that DMPK is not simply about the running of standard assays, as quickly and cheaply as possible, to churn out data that ultimately doesn’t inform on chemical design or aid in translation to the clinic. Of course, the DSTA cycle must be as efficient as possible, but it is the strategic input and the targeted investigation with expert interpretation which glues the discovery processes together to allow the best drug design. The starting point is the chemical structure, and the initial investigations are driven by it and guided by a DMPK expert so that the right study is done at the right time. This leads to key decision-making data generated to keep the project moving in the right direction or indicating that it is time to stop and move to new chemistry.
If you would like to speak to a member of the Sygnature Discovery DMPK team about any issues – from the design of early stage screening cascades, data interpretation and understanding how the body is treating your compounds, and ultimately dose prediction in humans – then contact us, today.

 Learn more about lead optimisation in our latest eBook: ‘Lead optimisation: Delivering molecules fit for preclinical development’
Learn more about lead optimisation in our latest eBook: ‘Lead optimisation: Delivering molecules fit for preclinical development’