Can small molecules compete with Ozempic and Wegovy and take a seat at the GLP-1 dinner table?
Over the last decade, Glucagon-like peptide-1 (GLP-1) based therapies have revolutionised diabetes treatment and are now set to massively impact the management of obesity. GLP-1 (7-36) is a natural peptide hormone that is secreted in response to food, activates the GLP-1 receptor (GLP-1R) and is a key regulator in glycaemic control. The main mechanisms behind effective glucose regulation are:
- Glucose-dependent insulin secretion
- Delayed gastric emptying and increased feeling of fullness (satiety)
- Suppressed glucagon release
GLP-1 has a half-life of a few minutes in vivo as it is cleaved by the dipeptidyl peptidase 4 (DPP-4) enzyme to yield the metabolite GLP-1 (9-36). A range of DPP-4 inhibitors (e.g. saxagliptin, sitagliptin) have been developed to inhibit the cleavage of GLP-1. These have proven to be clinically effective treatments for diabetes while being weight-neutral.
DPP-4-resistant peptide mimics of natural GLP-1 have been pioneered to maintain and boost the biological response of GLP-1R activation. Prominent examples such as liraglutide and semaglutide have proved effective in glycaemic control and reducing HbA1c, a key biomarker in diabetes.
Furthermore, GLP-1 receptor agonists improve CV risk factors, including body weight reduction, and lowered systolic blood pressure and LDL cholesterol. They may also have beneficial effects on the complications of diabetes such as diabetic nephropathy, since they are reported to reduce the ratio of albumin to creatinine in urine in patients with type 2 diabetes. Empirical evidence also suggests that while GLP-1 therapies may be useful in treating impulsive/addictive behaviours such as alcoholism and binge eating disorder, these therapies may have adverse effects, such as nausea, vomiting, and diarrhoea.
GLP-1 treatment for diabetes and obesity
Semaglutide, a GLP-1 receptor agonist, is the active ingredient in Ozempic for diabetes and in Wegovy for obesity. Clinical trials with Wegovy have reported up to 15% weight loss in some cases when combined with lifestyle intervention (reduced-calorie diet and increased physical activity). Semaglutide could be a significant tool in the weight loss battle for some, however popular media reporting of the “skinny jab” may raise unrealistic expectations of easy weight loss within the general public.
Orally dosed semaglutide has low bioavailability as a once-daily tablet and can have variable pharmacokinetics. The dosing recommendation is 30 minutes before the first food or beverage to maximise its effect. Hence, most patients opt for a once-weekly subcutaneous injection. However, injectable semaglutide requires refrigeration prior to first use and some patients are unable or unwilling to self-inject. Similar considerations may limit the utility of the injectable agents, tirzepatide (dual GLP-1 and glucose-dependent insulinotropic polypeptide [GIP] agonist), and retatrutide (triple glucagon, GIP, and GLP-1 receptor agonist).
In general, oral therapies are associated with improved convenience acceptance and compliance by patients, particularly when taking additional medications, as is often the case in the management of diabetes. Hence, a significant market opportunity exists for orally dosed small molecules with good bioavailability to address these needs.
Where peptides lead the way, small molecules are sure to follow. After the success of peptide agonists of GLP-1R (a class B G-protein coupled receptor) in the treatment of diabetes, drug discovery scientists saw the opportunity for small molecule agonists of GLP-1R. In addition, intensive research focused on biological targets which could stimulate GLP-1 release in the gut or pancreas, including GPR120, SSTR5, GPR40, TGR-5 and GPR119.
Small molecule GLP-1 agonists in the clinic
Orally dosed small molecule receptor agonists of GLP-1R such as danuglipron, lotiglipron and orforglipron (Figure 1) have been discovered and have progressed into clinical trials. These small molecules delivered substantial body weight loss (efficacy). For example, once-a-day orforglipron was associated with a 14.7% body weight loss after 36 weeks in overweight/obese patients. Danuglipron (dosed twice daily) and orforglipron (once daily) continue in clinical trials, while the clinical development of lotiglipron (once daily) was recently stopped due to elevated transaminase liver enzymes. Such elevations were not observed in the danuglipron program.
Figure 1
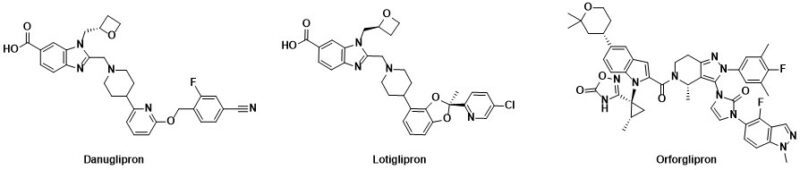
Potential for differentiation with small molecules
Detailed investigation of the small molecule GLP-1 receptor agonist danuglipron showed that, compared to natural GLP-1, the synthetic agonist showed differences in GPCR coupling to the cAMP and B-arrestin downstream biological pathways. Such differences are increasingly being viewed as important for biological understanding, with the potential for biological differentiation between small molecule and peptide agonists, and perhaps greater efficacy.
In addition, small molecule positive allosteric modulators (PAMs) offer the potential for differentiation based on efficacy or safety profile. Several reports now highlight small molecules that potentiate the action of the active peptide GLP- 1 (7-36). Also, small molecule PAMs of GLP-1 have been found that can potentiate the action of GLP-1 (7-36) and its metabolite GLP-1 (9-36), which retains some weak activity (EC50 = 1.8 μM, EMAX = 13%) for GLP-1R. This highlights that there could be more potential opportunities around positive allosteric modulation of the GLP-1 receptor.
Conclusion
So, can orally dosed small molecules get a seat at the GLP-1 dinner table? The jury is out at the moment, but their potential to be differentiated from injectable peptides is clear and seems likely to drive significant research into small molecule modulators of GLP-1 for the treatment of diabetes, obesity and perhaps addictive behaviours for many years to come.
