Exploring the Lifelines of the Brain: Autophagy and Lysosomal function at the Heart of Neurodegeneration
The human brain, a marvel of complexity and sophistication, is the epicentre of our consciousness, cognition, and overall well-being. It is an organ that never truly rests, continuously processing and orchestrating a symphony of activities. However, this extraordinary organ is not immune to the ravages of time and disease. Neurodegenerative diseases, a group of disorders characterised by the progressive degeneration of neurons, pose a significant challenge to human health and quality of life. Understanding the cellular mechanisms behind these disorders is vital for developing effective treatments and interventions.
Two cellular processes, autophagy and lysosomal function, have emerged as critical players in the pathophysiology of neurodegenerative diseases. Autophagy serves as the primary pathway for the selective elimination of unwanted cellular materials to maintain cellular equilibrium. This includes neurotoxic proteins such as beta-amyloid and Tau, associated with Alzheimer’s disease, alpha-synuclein related to Parkinson’s disease, and impaired organelles like mitochondria. The lysosome, often called the “stomach” of the cell, is essential in degrading and recycling cellular waste. It contains a mixture of enzymes capable of breaking down various biological molecules, including proteins, lipids, and carbohydrates. Autophagy and lysosomal function are closely intertwined, with autophagic cargo delivered to lysosomes for degradation.
Autophagy: The Cellular Cleanup Crew
The word autophagy means “self-eating,” and this is precisely what the process entails since it serves as a cellular “cleanup crew” removing damaged organelles, protein aggregates, and other cellular waste. Like all cells, neurons rely on autophagy to maintain their health and functionality. Dysregulated autophagy can lead to neuronal damage and death.
In neurodegenerative diseases, autophagy is crucial for clearing misfolded and aggregated proteins and regulates oxidative stress and mitochondrial health. Mitochondria, the cell’s energy powerhouses, are especially critical in neurons which have high energy demands. Autophagy selectively removes damaged mitochondria through a process known as mitophagy, ensuring that only healthy mitochondria continue to produce energy. Dysfunctional mitochondria are a source of oxidative stress and a common feature of neurodegenerative diseases.
The role of autophagy is further emphasised in Parkinson’s disease, where mutations in genes like PARK2 are associated with damaged mitochondria and alpha-synuclein accumulation. Dysregulated autophagy can also lead to neuroinflammation, a feature of several neurodegenerative diseases.
Lysosomal Function: The Cellular Digestive System
Lysosomes are crucial to the degrading and recycling components of the cell and in receiving autophagic cargo for degradation. When proteins and organelles earmarked for degradation come into close contact with lysosomal enzymes, they are broken down into their basic components such as amino acids, fatty acids and sugars. The cell then recycles these components to meet its energy and building block needs.
Once lysosomal function falters, the cell becomes cluttered with undigested material. This accumulation is a hallmark of lysosomal storage disorders, a group of inherited diseases resulting from mutations in lysosomal genes. In diseases like Niemann-Pick disease, lysosomal dysfunction leads to the accumulation of lipids, including neurons. Moreover, such accumulation can disrupt other cellular processes, ultimately causing neurodegeneration. Although lysosomal storage disorders are not classified as neurodegenerative diseases, understanding lysosomal function is essential because these disorders often present with neurological symptoms.
The Interplay of Autophagy and Lysosomal Function
Autophagy and lysosomal function are intimately interconnected. This partnership is essential for maintaining cellular health, and its breakdown can have disastrous consequences. When the autophagic-lysosomal pathway is compromised, it creates a vicious cycle. Autophagy cannot efficiently clear aggregated proteins or damaged organelles, and lysosomes become overwhelmed with undigested material. This leads to cellular stress and inflammation, further compromising cellular function and contributing to neuronal death.
The first step of autophagy and content degradation is the formation of autophagosomes (Figure 1). Autophagosomes are double-membraned structures that encapsulate cellular components to be degraded. Damaged or unwanted cellular components, such as protein aggregates, organelles, or lipids, are then “targeted” for degradation by a protein called p62. Autophagosomes grow and expand, engulfing the selected cargo within their double membranes. Autophagosomes fuse with lysosomes (autolysosomes) and expose the cargo to lysosomal enzymes. The content is finally broken down into its essential components, such as amino acids, fatty acids, and sugars, and the components are recycled and waste products eliminated.
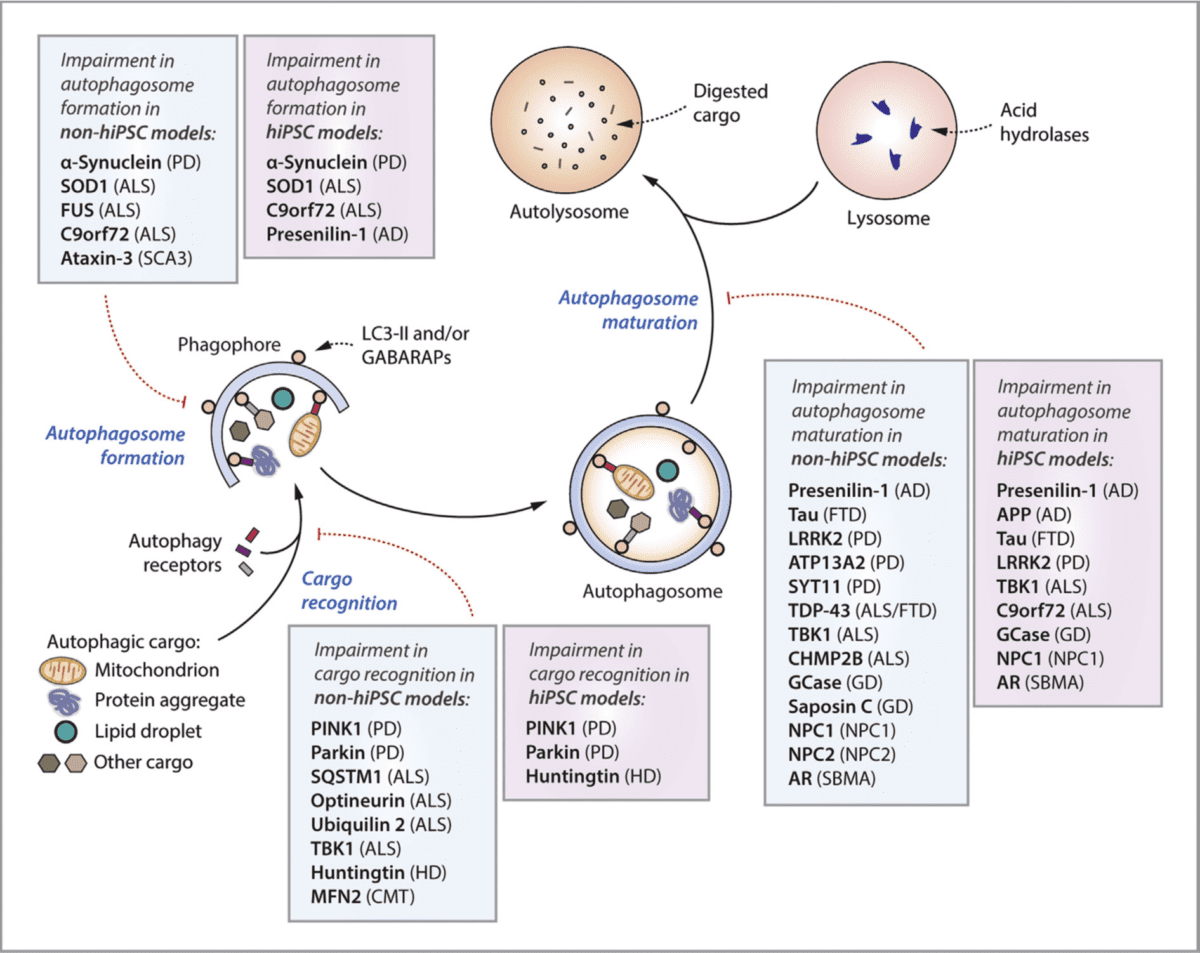
Figure 1: Schematic representation of autophagy and lysosomal steps, and their relationship to neurodegenerative diseases. Reference: Seranova E, Palhegyi AM, Verma S, Dimova S, Lasry R, Naama M, Sun C, Barrett T, Rosenstock TR, Kumar D, Cohen MA, Buganim Y, Sarkar S. Human Induced Pluripotent Stem Cell Models of Neurodegenerative Disorders for Studying the Biomedical Implications of Autophagy. J Mol Biol. 2020 Apr 3;432(8):2754-2798. doi: 10.1016/j.jmb.2020.01.024.
Assays to interrogate Lysosomal Function
Currently, multiple endeavours aim to measure lysosomal quantification, activity, and function. These efforts involve diverse imaging platforms and cells from healthy individuals and those with neurodegenerative diseases. The assessment includes evaluating changes in several lysosomal markers such as TMEM175, LAMP-1, and LysoTracker™, as well as measuring enzyme activity within the lysosome (Figure 2).
- TMEM175 is implicated in various diseases, including Parkinson’s disease.
- LAMP-1 is a glycoprotein that plays a crucial role in the structure and function of lysosomes, and it is primarily found on the inner surface of the lysosomal membrane.
- LysoTracker™ (fluorescence probe) measures the acidity inside lysosomes and is a marker of both lysosomal content and function.
- MagicRed® (fluorescence probe) measures the activity of the intracellular enzyme Cathepsin B.
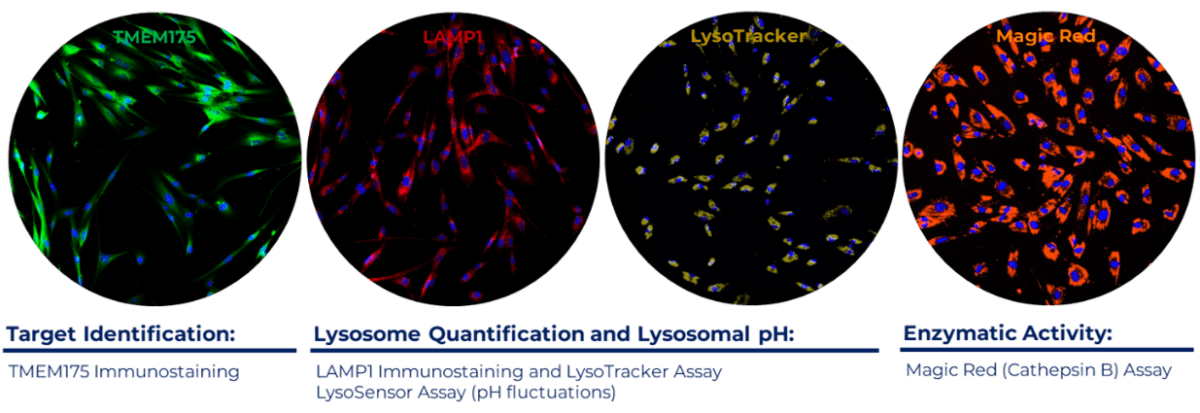
Figure 2: Representative images of control fibroblasts stained with TMEM175, LAMP-1, Lysotracker™ and MagicRed®. Cells were grown overnight in the presence of serum and incubated with antibodies against TMEM175 and LAMP-1 and with the fluorescent probes LysoTracker and MagicRed ®.
Assays to cross-examine Autophagy
Equally, several imaging platforms can be used for monitoring autophagy dysfunction and modulation. Control and diseased cells can be stained using primary antibodies against p62 to investigate autophagic flux, as it ” targets” the proteins and organelles to be degraded (Figure 3), and LC3-I and LC3-II to determine autophagosome formation and maturation. Importantly, levels of p62 and LC3 can be also monitored by Jess-based western blot.
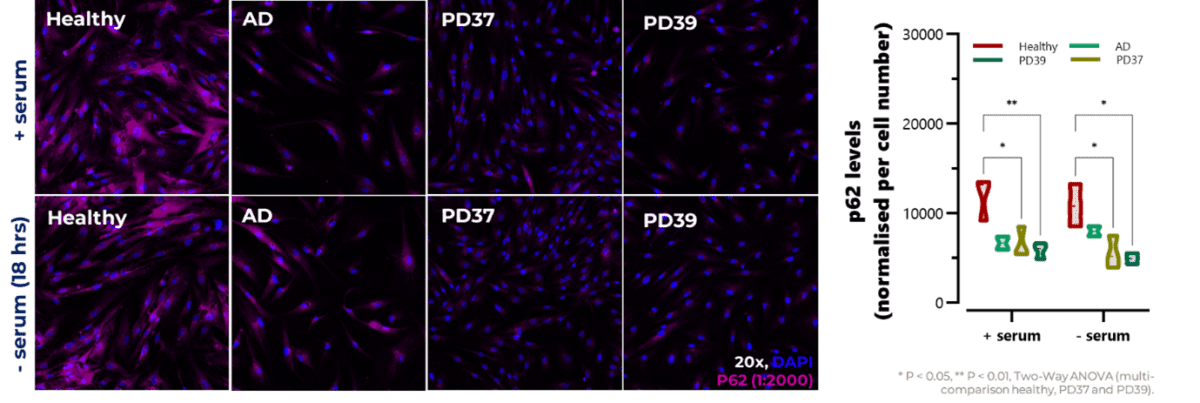
Figure 3: Representative images of control (healthy) and diseased fibroblasts incubated with primary antibody against p62. Cells were grown overnight in the presence or absence of serum. Data was normalised by cell number (per image). AD: Alzheimer`s Disease; PD37: Parkinson`s Disease (donor 1); PD39: Parkinson`s Disease (donor 2).
Conclusion: The Promise of Autophagy and Lysosomal Function
Drugs that modulate autophagy and lysosomal function hold promise as therapeutic targets. However, it is essential to highlight that achieving specificity for autophagy and lysosomal modulation is challenging. Many cellular processes and pathways intersect with autophagy, and drug development must target the intended pathway without causing unintended side effects.
Employing multi-endpoint autophagy and lysosomal function assays may help unravel the underlying biology of neurodegenerative diseases. This could prove invaluable in advancing the most promising new compounds for developing new therapies.
