Caco-2 Permeability
The human colon epithelial cancer cell line, Caco-2, is used as a model of human intestinal absorption of drugs. This model is suitable to test compound suitability for oral dosing, predict intestinal permeability and investigate drug efflux.
When cultured as a monolayer, Caco-2 cells differentiate to form tight junctions between cells to serve as a model of passive diffusion of compounds across the monolayer. Various uptake and efflux transporters are expressed in Caco-2 cells.
Differentiated and polarized Caco-2 cells (21-day system) are plated on a 96-transwell permeable system as a single monolayer to allow for automated high throughput screening of compounds. Drug transport is assessed in both directions (apical to basolateral (A-B) and basolateral to apical (B-A)) across the cell monolayer (Figure 1). The buffer used for the assay does not include HEPES, to minimise the inhibitory effect on uptake transporters (Luo et al., 2010).
Test compound concentrations are quantified using a calibration curve following analysis by LC-MS/MS, and the apparent permeability coefficient (Papp) and efflux ratio of the compound across the monolayer are calculated. The efflux ratio is used as an indicator of active efflux.
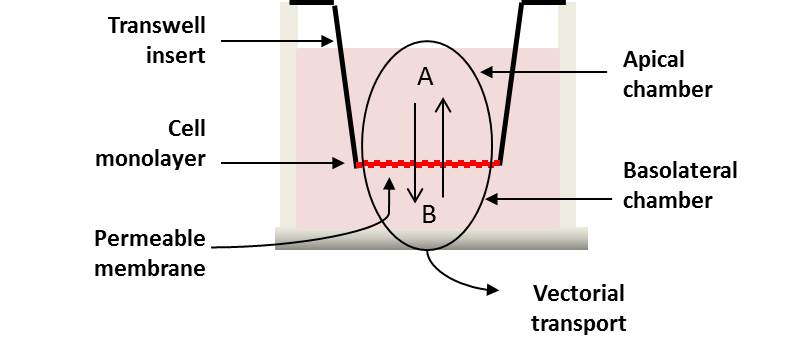
Figure 1 Schematic illustrating the Transwell of CacoReady™ Multiwell Insert
