Acute feeding studies
Acute feeding studies provide an excellent screening platform to quickly understand the hypophagic potential of a novel drug.
These assays are typically performed in lean mice or rats but can also be performed in a variety of animal models, such as the DIO mouse. Following acute administration, the effect of the novel test drug on food intake is evaluated. Where a delayed onset of drug effect is forecast, dosing can continue for up to 7 days.
An example of an acute study set up is as follows, for mice and rats:
- Drugs can be administered by various routes (e.g. po, sc, ip, iv)
- Mice or rats are acclimatised to palatable wet mash (4 h/day). Drugs are administered before mash and food intake is measured subsequently.
- Rats are maintained under reverse-phase lighting with free access to normal, powdered rat chow. Drugs are given at the onset of the dark photoperiod and food intake is measured for up to 24 h.
- Determination of ED50 values for inhibition of food intake
- Blood sampling for pharmacokinetic analysis (at the end of studies or from satellite groups of animals)
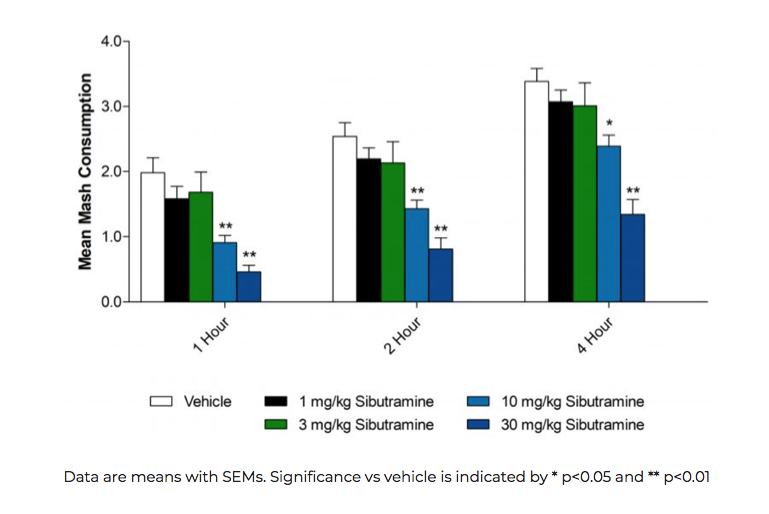
Sibutramine reduces acute food intake (mean mash consumption) in a dose-dependent manner in mice
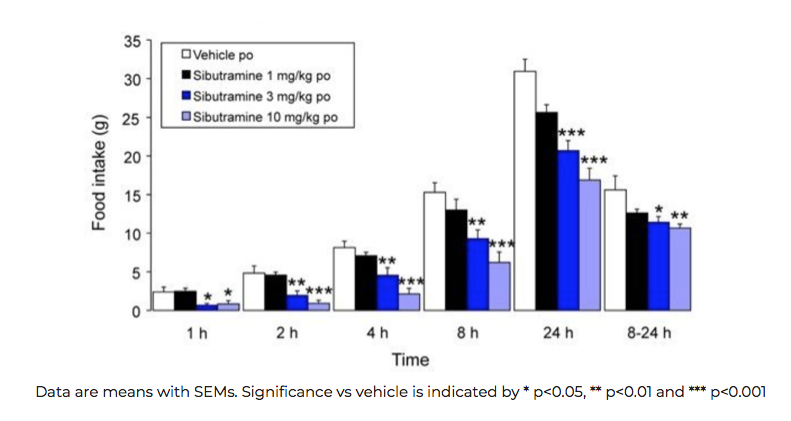
Sibutramine reduces acute food intake in a dose-dependent manner in rats
Get in touch if you want more information, or browse our models for metabolic diseases.
