Unlocking the Power of Phenotypic Screening in Drug Discovery
In the vast landscape of drug discovery, where the journey from concept to cure is often fraught with challenges and uncertainties, researchers constantly seek innovative approaches to accelerate the process. Among these approaches, phenotypic screening stands out as a powerful technique with the potential to revolutionize the discovery of new therapeutic agents and deliver mainly first-in-class drugs.
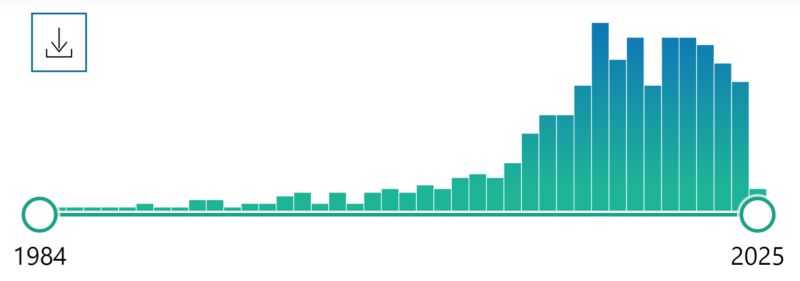
Figure 1: Graph showing PubMed search volumes for ‘phenotypic screening’ by year.
PubMed – “Phenotypic screen” query hits by year.
In recent times, drug discovery has relied heavily on target-based approaches, where molecules are designed to interact with specific molecular targets implicated in disease processes. While this strategy has led to remarkable breakthroughs, it also has limitations, particularly when the underlying molecular mechanisms of a disease are not fully understood or when targeting a single pathway fails to yield the desired clinical outcomes as manifested by the high late-stage failure rate of drugs in development due to lack of efficacy1.
Phenotypic screening offers a complementary approach by focusing on cells’ or organisms’ observable characteristics, or phenotypes, in response to drug treatment. Instead of starting with a predefined molecular target, researchers expose disease-relevant cells or model organisms to libraries of compounds and observe changes in phenotype, such as cell morphology, viability, or function. To increase the likelihood of a phenotypic hit, these compound libraries can be crafted using a broad range of chemotypes from natural products, peptides and compounds with known bioactivity. By analyzing these phenotypic changes, researchers can identify compounds that modulate the disease phenotype, even if the underlying exact molecular mechanism of action is initially unknown2,3.

Figure 2: Table showing the advantages of phenotypic screening as a powerful technique.
Sygnature’s HIT SYNERGY platform accesses a wide range of Hit ID technologies from phenotypic HTS screening to biophysical fragment screening.
One of the most significant advantages of phenotypic screening is its unique ability to uncover novel drug candidates and pathways that may have been overlooked using traditional target-based approaches, especially cellular processes that can’t be recapitulated in isolated molecular assays. By capturing the integrated effects of compounds on cellular systems, phenotypic assays have the potential to reveal unexpected biological interactions and identify compounds with diverse mechanisms of action. This can lead to the discovery of drugs with unique modes of action or the repurposing of existing compounds for new indications, ultimately expanding the therapeutic options available to patients. For example, Risdiplam, a new drug with a new mechanism to treat spinal muscle atrophy, was identified using a mechanism-agnostic cell system4.
Figure 3: Skeletal structure of Risdiplam, a novel drug identified using a mechanism-agnostic cell system to treat spinal muscular atrophy.
Moreover, phenotypic screening has proven particularly valuable in the early stages of drug discovery when researchers are still exploring the biology of a disease. By examining the phenotypic effects of compounds in disease-relevant cellular models, researchers can gain insights into the underlying disease mechanisms and identify new therapeutic targets. This knowledge-driven approach not only accelerates the drug discovery process but also increases the likelihood of clinical success by focusing on biologically relevant pathways.
In recent years, advances in high-throughput screening technologies, imaging techniques, and computational analysis have further enhanced the power and efficiency of phenotypic screening. Automated platforms can now rapidly screen thousands of compounds against complex cellular models, allowing researchers to identify promising candidates more quickly and cost-effectively than ever before. Additionally, sophisticated image analysis algorithms powered by machine learning and artificial intelligence enable the quantification of subtle phenotypic changes, providing valuable insights into drug mechanisms of action and cellular signalling pathways.
Despite its promise, phenotypic screening also presents challenges, particularly in data interpretation and validation. Since phenotypic assays capture complex biological responses, distinguishing between compounds’ direct and indirect effects can be challenging. Furthermore, phenotypic hits can operate via polypharmacology mechanisms which can be extremely challenging to optimise. Validating hits from phenotypic screens and elucidating their mechanism of action is, therefore, very important and often requires additional experiments and rigorous, innovative follow-up studies. To identify which target(s) a hit engages with, a range of “deconvolution” platforms exist5, from photo-affinity labelling with chemical probes6 to more indirect methods like chemogenomic profiling7.
Sygnature Discovery has access to a network of hit deconvolution technologies that can validate hits and determine their on- and off-target profiles.
Case Study
For one recent target deconvolution project at Sygnature Discovery, a client was interested in identifying novel targets that contribute to the efficacy of a known drug. A proteome-wide CETSA® (Cellular Thermal Shift Assay) identified candidate targets that we investigated individually for functional significance using small molecule and CRISPR modulation. We showed that one of these targets mediates a novel pharmacology that contributes to the desired overall cellular efficacy and, moreover, had a different SAR to other targets that contribute to the drug’s efficacy.
The potential of phenotypic screening to drive innovation in drug discovery is undeniable. By embracing a phenotype-first approach, researchers can uncover new therapeutic opportunities and novel molecular mechanisms, gain deeper insights into disease biology, and ultimately deliver more effective treatments to patients. As technology continues to evolve and our understanding of disease mechanisms expands, phenotypic screening will undoubtedly remain a key aspect of modern drug discovery, unlocking the full potential of the vast chemical space available to address unmet medical needs.
Bibliography
- 90% of drugs fail clinical trials. https://www.asbmb.org/asbmb-today/opinions/031222/90-of-drugs-fail-clinical-trials.
- Vincent, F. et al. Phenotypic drug discovery: recent successes, lessons learned and new directions. Nat. Rev. Drug Discov. 21, 899–914 (2022).
- Gerckens, M. et al. Phenotypic drug screening in a human fibrosis model identified a novel class of antifibrotic therapeutics. Sci. Adv. 7, eabb3673 (2021).
- Naryshkin, N. A. et al. SMN2 splicing modifiers improve motor function and longevity in mice with spinal muscular atrophy. 7.
- Tabana, Y., Babu, D., Fahlman, R., Siraki, A. G. & Barakat, K. Target identification of small molecules: an overview of the current applications in drug discovery. BMC Biotechnol. 23, 44 (2023).
- Kotake, Y. et al. Splicing factor SF3b as a target of the antitumor natural product pladienolide. Nat. Chem. Biol. 3, 570–575 (2007).
- Pries, V. et al. Target Identification and Mechanism of Action of Picolinamide and Benzamide Chemotypes with Antifungal Properties. Cell Chem. Biol. 25, 279-290.e7 (2018).
Find out more about Sygnature Discovery’s powerful hit ID platform, HIT SYNERGY, which integrates multiple advanced screening technologies to rapidly generate high-quality, structurally diverse hits, tailored to today’s most challenging new target classes.
