Targeting Neuroinflammation: Using the Right Tools/Models to Decipher the Brain’s Immune Response and Evaluate Novel Drug Candidates
Substantial progress has been made in our understanding of neuroimmune interactions and it is no longer believed that the brain is an immune-privileged site. Our improved knowledge of neuroinflammatory mechanisms has not only unveiled the role of neuroinflammation in the development of neurological and psychiatric disorders but has also revealed novel targets associated with neuroinflammation.
Currently, there are no clinically approved drugs that demonstrate therapeutic benefit in a CNS disease by reversing or dampening neuroinflammation. Therefore, there is a need to identify novel therapeutic targets that reduce neuroinflammation and lead to therapeutic efficacy. Within a drug discovery and development pipeline, druggable targets can be identified through Hit-to-Lead campaigns to select the best molecules to take forward in development and to understand neuroinflammation.
Although key aspects of neuroinflammation can be modelled in vitro and in vivo using pro-inflammatory agents, it’s important to use the most suitable models. These models are not only to investigate neuroinflammatory processes in the CNS but also to elucidate the mechanism of action and efficacy of potential drug candidates in the neuroinflammation space.
Microglia: Reproducing their Functions In Vitro
Deciphering the brain’s immune response requires the use of the right tools. Recent focus has turned to microglia as they are now recognized as being key cellular mediators in both acute and chronic neuroinflammatory processes.
Microglia, the brain’s resident macrophages are responsible for phagocytosis and the elimination of cell debris and protein aggregates, as well as the removal of other antigens that may endanger the brain.
Microglia also secrete many soluble factors, including cytokines that contribute to various aspects of the immune response. Replicating all these functions in vitro can be a challenge, but a range of in vitro models with different advantages and disadvantages are necessary for any drug discovery platform. Models such as:
Immortalised cell lines:
Rodent (BV2, N9) and human (HMEC3) cell lines are clearly an excellent option from a screening perspective, but these cells fail to recapitulate the characteristics of adult rodent and human microglia, respectively.
Primary rodent microglia:
Isolating primary microglia from neonatal or adult rodent brains offers a more realistic model of microglia. Isolation of primary mouse/rat microglia is time-consuming, and culture of these cells is limited with few numbers of cells obtained per animal. However, depending on the question and throughput required primary microglia can be a ‘fit for purpose’ model system.
Humanised models of microglia:
When considering humanised models, human iPSC-derived microglia are an excellent alternative to rodent microglia. Albeit, there are technical difficulties and ethical considerations. Yet, iPSC-derived microglia might still be amenable to screening campaigns, and they also offer the possibility to explore distinct phenotypes by using iPSCs derived from patients with specific neurological disorders. Recent publications have also referred to monocyte-derived microglia cells (MDMi), and it has been shown that MDMi recapitulates a microglia phenotype and function.
When selecting an in vitro microglia model, several factors need consideration. For example, are we looking into a high-throughput screening (HTS) campaign, in which millions of cells will be needed? or later in the Drug Discovery process when a more realistic model is necessary for mechanistic understanding and validation of a drug candidate?
Clearly, there is a continuum between these two extremes, but functional assays and tools that you will need include:
- Identification of microglia surface markers (i.e., IBA1, TMEM119, P2YR12) and morphology using immunofluorescence/live cell imaging.
- Pro-inflammatory agents to trigger microglia activation (such as, lipopolysaccharide, LPS) and measurement of activation markers (CD68, TREM2).
- Gene expression (e.g., Nos-2, Arginase-1).
- Cytokine release (e.g., IL-6, TNF-α).
- Phagocytosis assays using E. Coli or brain cell debris using live cell imaging.
- Calcium release.
- Oxygen consumption.
In Vivo LPS Model for Evaluation of the Efficacy of Novel Anti-Inflammatory Drugs
As a Drug Discovery project progresses, it is equally important to use the most appropriate in vivo model for evaluating either the mechanism of action or efficacy of a novel compound in augmenting neuroinflammation.
Lipopolysaccharide (LPS) is a potent endotoxin and an agonist of Toll-like receptor 4 (TLR-4). Within the CNS, TLR-4 are primarily expressed on microglia, which once activated, produce pro-inflammatory cytokines, such as TNF-α and IL-1β. Furthermore, LPS induces a complex array of behaviours and several of these symptoms are thought to be very similar to the clinically relevant symptoms of neurodegenerative disease in humans. Therefore, the administration of LPS either peripherally or centrally to rodents is frequently used to evaluate certain aspects of neuroinflammation.
However, when establishing this model, careful consideration is required for the choice of serotype, dose, route and time of administration of LPS.
Efficacy/Pharmacodynamic endpoints after LPS challenge in-vivo include:
- Behaviour in the Morris water maze and passive avoidance test to investigate cognitive impairment such as occurs in Alzheimer’s disease.
- Gene expression (e.g., IL-1β, IL-6, TNF-α).
- Cytokine release (e.g., IL-6, TNF-α).
- Identification of microglia markers (e.g., IBA1, TSPO, P2X7) and morphology using immunohistochemistry
- Expression of TSPO using [3H]DPA-714 autoradiography
In summary, the use of in vitro microglia models and administration of pro-inflammatory agents, such as LPS, to induce neuro-inflammation both in in vitro and in vivo are powerful approaches to interrogating neuroinflammation. When combined with readouts such as pro-inflammatory biomarkers e.g. cytokines and IB1A, they can have translational validity enabling the development of targeted therapeutic interventions in patients where neuroinflammation is driving the disease.
Neuroinflammation presentations at Society for Neuroscience (SfN), Washington, November 11-15, 2023.
At the SfN meeting, Sygnature Discovery will be showcasing some of the in vitro and in vivo capabilities in the neuroinflammation space with two presentations.
In Vitro Microglia Models as a Screening Platform to Support Neuroinflammation Drug Discovery Programs (Presentation: Leite, et al.)
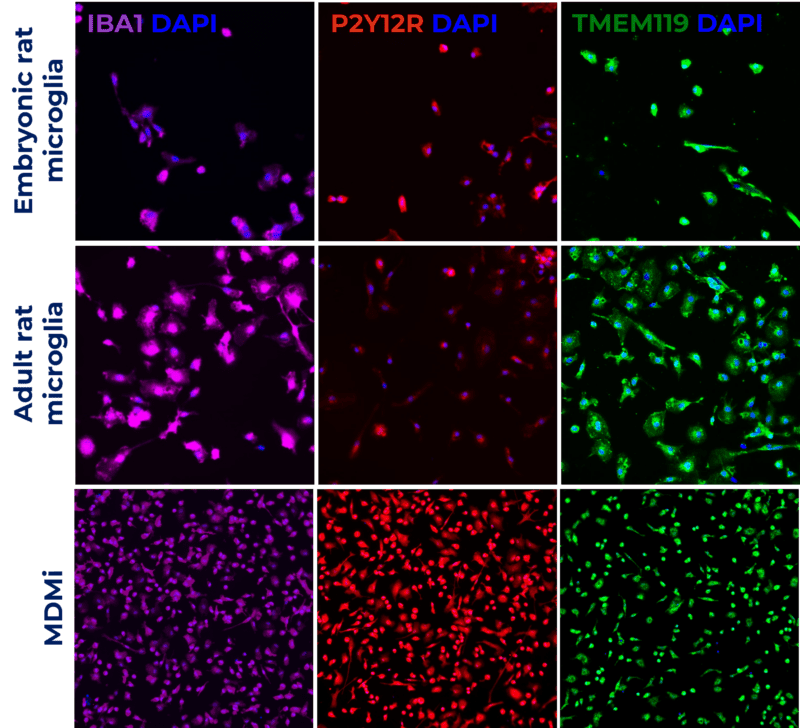
This poster showcases validated in vitro models: rat embryonic and adult microglia and human monocyte-derived microglia (MDMi). Models were characterised by cell surface markers (IBA1, P2Y12R and TMEM119), and the ability to recapitulate microglia phenotype and function by measuring phagocytosis.
Hear more about this topic at Dr Diana Leite poster presentation on Monday 13th November 8-12pm. Poster: PSTR203.05.
Preventative Effects of Dexamethasone in Lipopolysaccharide-Induced Neuroinflammation (Presentation: Jagger, et al.)
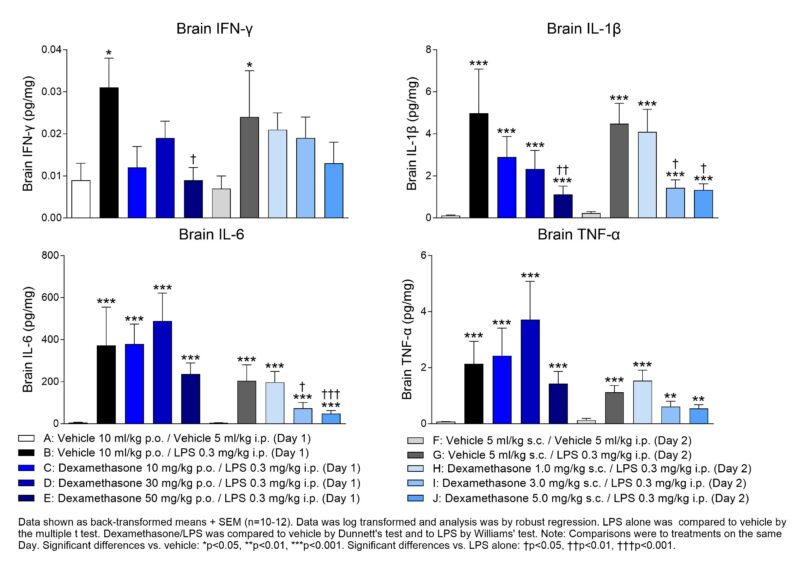
This poster showcases a validated in vivo model of neuroinflammation following peripheral LPS administration to C57BL/6 mice. Measurement of key pro-inflammatory biomarkers in brains 4 hr following LPS challenge and treatment with the anti-inflammatory drug dexamethasone provides a useful approach for testing novel anti-inflammatory drugs in early drug discovery.
Delve deeper into this topic at Dr Liz Jagger’s poster presentation on Tuesday 14th November 8-12 pm. Poster: PSTR332.28
Interested to learn more about how Sygnature Discovery can assist with your CNS drug discovery screening cascades? These cascades enable us to follow the molecule further. Meet members of our team at the Society for Neuroscience (SfN) at Booth 932, to hear more about Sygnature Discovery’s capabilities.
