High throughput screening of inverse agonists
Screening GPCR inverse agonists via high throughput methods offers substantial value to drug discovery projects, however, developing high-quality screening assays can be challenging. Here we discuss a different approach to inverse agonist screening.
Therapeutic benefit of GPCR inverse agonists
G-protein-coupled receptors (GPCRs), the largest family of cell surface receptors, detect a wide variety of signalling molecules, including neurotransmitters, hormones, ions, and even photons.
Dysfunction of GPCR signalling is linked to diseases including cancer, obesity, fertility disorders and immune-related conditions. Many drugs function by modulating GPCR activity, with around 30% of FDA-approved drugs in 2017 having a GPCR target (Hauser, 2017).
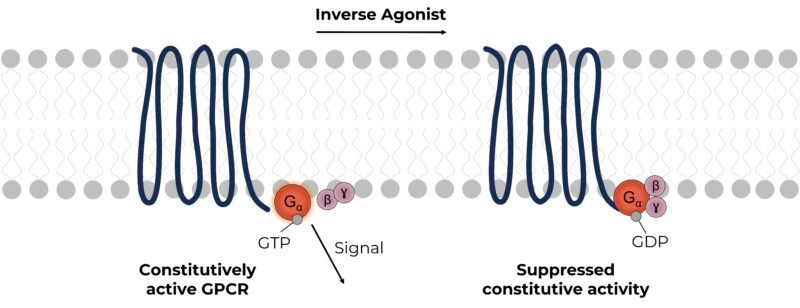
Molecules that activate GPCRs are termed agonists, and this activity can be inhibited by molecules referred to as antagonists. In some cases, GPCRs are constitutively active, in the absence of an agonist and it takes an inverse agonist to reduce this basal activity. Many wild-type receptors are constitutively active, but their activity can also be exacerbated by mutations.
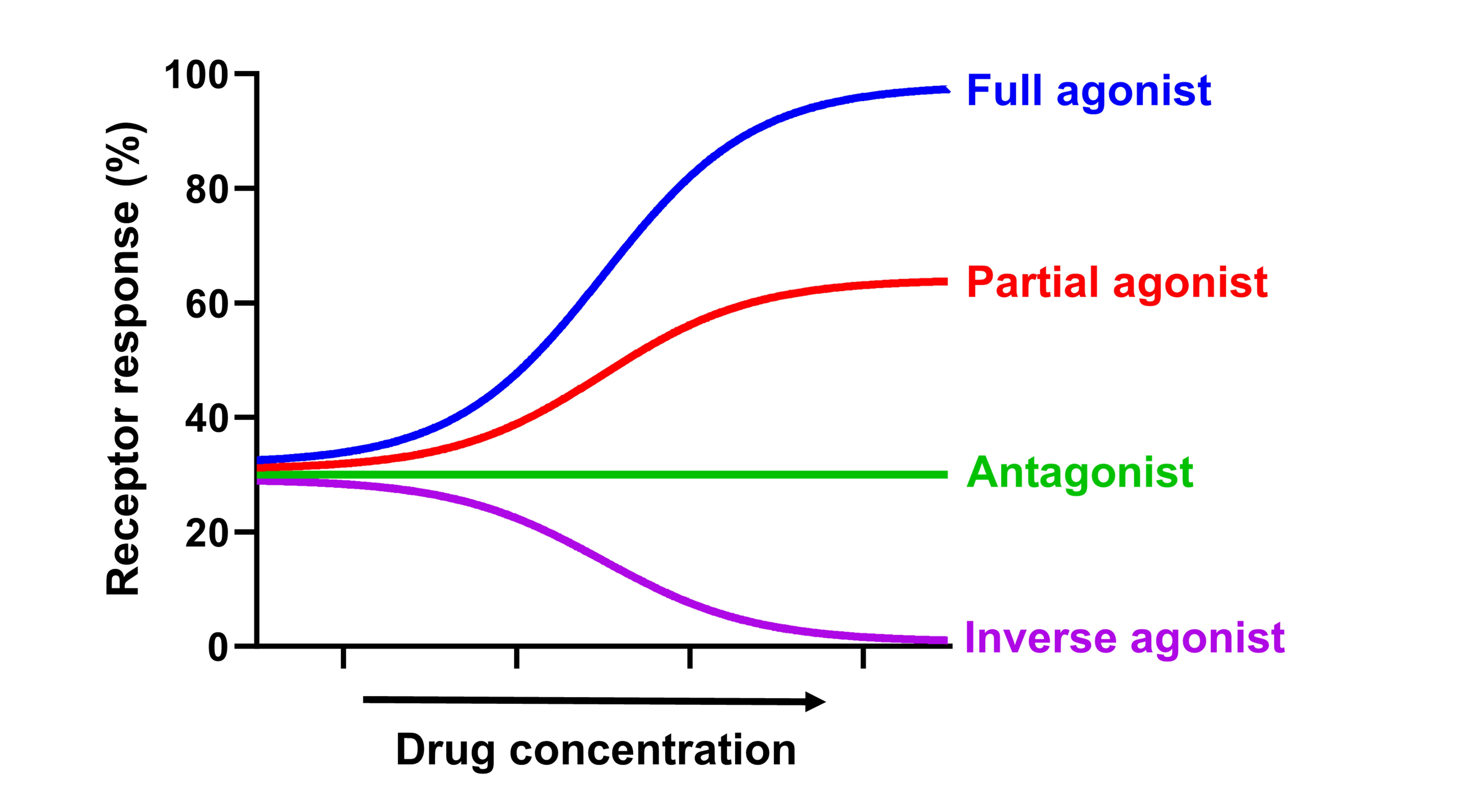
Inverse agonists have great therapeutic potential in modulating the activity of constitutively active GPCRs. For example, Pimavanserin (marketed as Nuplazid) an inverse agonist of the serotonin type 2A receptor, is used in the treatment of hallucinations and delusions caused by Parkinson’s disease (Cruz, 2017).
Inverse agonists are notoriously difficult to screen for
Despite their therapeutic potential, screening for inverse agonists is notoriously challenging. Primarily because inverse agonist assays are limited by very narrow signal windows caused by the low constitutive activity of GPCRs. The difficult challenge of developing high-quality screening assays has limited the number of inverse agonist drugs currently in development.
We were tasked with running an HTS to identify inverse agonists of a GPCR. To achieve this, we developed an inverse agonist assay using the IP-One Gq kit from CisBio. This assay was suitable for potency screening, however, unsuitable for HTS. The low constitutive activity of the receptor resulted in significant background noise and a narrow signal window. Additionally, the assay involved a 3-day protocol with transient transfection, rendering it incompatible with timely HTS.
A novel approach to screening for inverse agonists
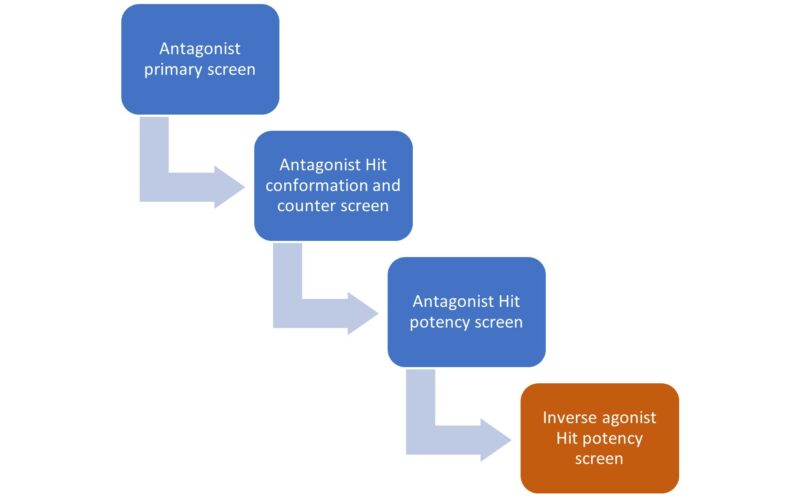
We have developed a novel approach to identifying inverse agonists using HTS. Instead of directly screening for inverse agonists, we ran an HTS looking for antagonists of our target GPCR. Subsequently, we profiled the identified antagonist compounds using an inverse agonist assay to pinpoint inverse agonists among the antagonist hits.
Inverse agonists behave as antagonists in the presence of an agonist-stimulated receptor and therefore will be identified as hits in an antagonist screen. Notably, several drugs like antihistamines and β-blockers were initially recognised as receptor antagonists before being reclassified as inverse agonists.
Screening in antagonist mode allows us to increase the assay window to a level suitable for HTS, which would not have been possible with an inverse agonist assay. This inverse agonist screening approach also offers the advantage of identifying both antagonists and inverse agonists, providing valuable SAR information useful for the Hit-to-Lead stage of the discovery process.
Download your free eBook: Successful In Vitro Strategies for Drug Discovery
Learn more about optimal cascade design, hit-to-lead phase insights and lead optimization strategies.
The outcome
We screened 200,000 compounds from our Lead Finder and Prism libraries in the antagonist assay. After triaging the hits through hit confirmation and counter screen stages we selected active compounds for subsequent inverse agonist profiling.
Of these 61% showed dose-dependent activity in the inverse agonist assay and are ready to be carried forward to the next stage of our drug discovery pipeline.
In summary
Developing robust HTS assays for inverse agonists is challenging. However, our approach, initially screening for antagonists and subsequently profiling the hits to identify inverse agonists, offers a viable alternative to direct inverse agonist screening.
HTS is costly, and each compound is initially screened with just a single data point. The assays must be of high quality, developed and validated to the highest standards, to ensure they are robust and reproducible to give the best chance of success for a project.
Inverse agonists are one of many difficult HTS targets, which are discussed in our ‘Hitting difficult targets’ blog.
About the authors
Dr Frank Baron
Frank Baron is a Senior Scientist within the Bioscience department with a PhD in Molecular Biology and 3 years of experience in Integrated Drug Discovery and High Throughput Screening.
Dr Denise Swift
Denise Swift is a senior Principal Scientist and HTS group leader. She has 20+ years of experience in the Biopharma industry, gained predominantly at Sygnature Discovery and AstraZeneca in one of their HTS centres where she was responsible for developing assays and running HTS.
References:
Cruz MP. Pimavanserin (Nuplazid): A Treatment for Hallucinations and Delusions Associated With Parkinson’s Disease. P T. 2017 Jun;42(6):368-371. PMID: 28579723; PMCID: PMC5440097.
Hauser AS, Attwood MM, Rask-Andersen M, Schiöth HB, Gloriam DE. Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov. 2017 Dec;16(12):829-842. doi: 10.1038/nrd.2017.178. Epub 2017 Oct 27. PMID: 29075003; PMCID: PMC6882681.

