Advancing alternative modalities – ASMS screens rapidly find novel ligands agnostic to binding site
This case study demonstrates how ASMS screening using our compound collections can accelerate discovery timelines while delivering high-quality, differentiated hit matter for drug discovery programmes.
Affinity Selection Mass Spectrometry (ASMS) is a versatile, label-free screening technology that works in solution and is applicable to a broad range of biological targets—including challenging non-protein targets like nucleic acids. Affinity-based screening is a valuable strategy in early drug discovery because it directly identifies compounds that bind to a biological target, regardless of whether they modulate its activity. This enables the detection of diverse binding interactions—including allosteric or cryptic site engagement—that may be missed by conventional activity-based screens. As a result, affinity screening broadens the landscape of potential hits and opens up novel therapeutic mechanisms. ASMS excels in identifying novel ligands and starting points for drug discovery, including allosteric modulators and induced proximity therapeutics. Unlike DNA-encoded libraries (DELs), ASMS enables immediate downstream work from DMSO or solid-stock without the delay of resynthesis, streamlining the hit validation process. It is especially well-suited to cases where traditional activity-based high-throughput screening (HTS) may fall short due to a challenging orthosteric site, offering an efficient route to tool compounds and new lead matter. With ASMS, even large, complex targets — like protein-DNA complexes — can be screened rapidly, expanding ligand discovery to challenging modalities that other techniques struggle to address.
Screening an Epigenetic Enzyme Target with ASMS
In a recent project, we applied ASMS to a soluble enzyme involved in epigenetic regulation — a target class linked to multiple diseases. Our team expressed and purified the wild-type protein in milligram quantities, supporting both ASMS screening and orthogonal validation efforts.
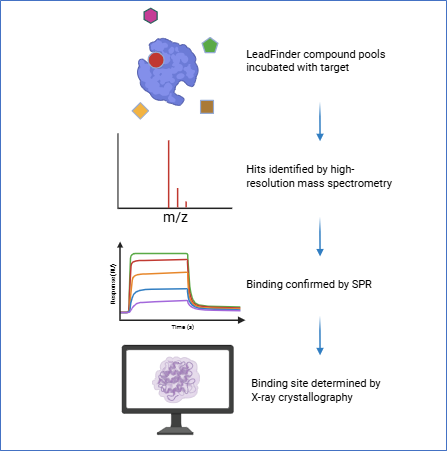
The LeadFinder compound library, comprising 150,000 chemically diverse small molecules, was compressed for screening using carefully constructed pools of 300 compounds. To ensure unambiguous hit identification, no two compounds within a pool share the same molecular formula. Pool creation was tracked using Titian Mosaic and executed with HighRes Biosolutions automation on our HTS platform.
Screening was conducted using the automated ligand identification system (ALIS) method on our Agilent 2DLC-TOF mass spectrometry system. Hits identified by molecular formula were then cherry-picked, dispensed individually using our automated system, and retested in singleton assays. Confirmed hits were progressed to orthogonal validation workflows.
Uncovering Novel Binding Modes
From the 150K compound screen, 71 hits were identified (0.048% hit rate), with 24 compounds confirmed in follow-up testing (0.016% confirmed hit rate). These hit rates are consistent with expectations for ASMS using an unbiased chemical library.
Next, we evaluated binding of the 24 confirmed compounds via surface plasmon resonance (SPR). Several showed concentration-dependent binding to the target protein, supporting direct interaction.
Pushing forward, we applied our in-house crystallography capabilities to crystallize the target protein and soak with selected ASMS hits. The crystal structures revealed two previously uncharacterised binding pockets, distinct from the active site — highlighting ASMS’s potential to uncover ligands with novel binding modes and providing new opportunities for structure-based drug design.
Accelerating Hit Identification with ASMS
By enabling rapid screening of a diverse chemical library, ASMS delivered confirmed hits within 6 weeks. Notably, some of these hits showed novel binding modes—offering promising new starting points for therapeutic intervention for a target with a challenging orthosteric site.
