Neuroplasticity and Neuroinflammation at AD/PD 2023: Sygnature Discovery Insights
So, another AD/PD meeting is over – the International Conference on Alzheimer’s and Parkinson’s Diseases and Related Neurological Disorders. This one in Gothenburg with over 4000 delegates and hundreds of posters. The buzz was tangible given the breakthrough with Leqembi for Alzheimer’s disease (AD) – great news for the field. And just as I write there is exciting news on donanemab in a Phase 3 trial for AD.

Dr Max Mirza is the VP of Neuroscience at Sygnature Discovery.
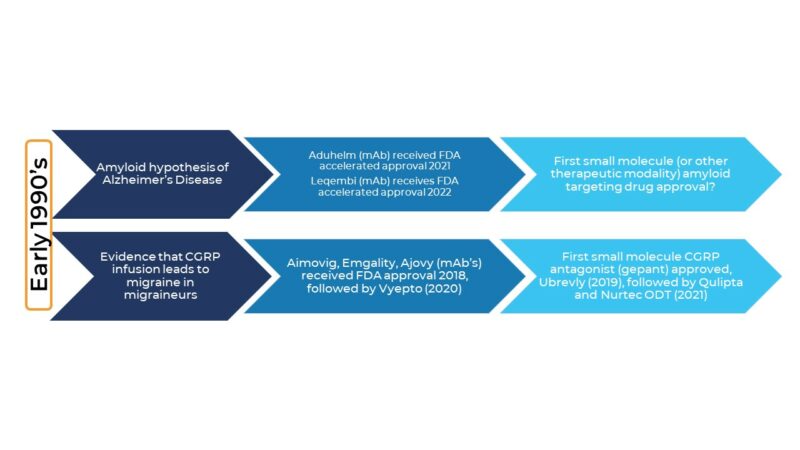
The timescale from the initial amyloid hypothesis of AD to the first therapeutic drug is not that dissimilar to the timescale for the first CGRP migraine therapies to hit the market after CGRPs role in migraine gained traction. Clearly, the challenges were different, but it shows you need to be in this for the long haul. Another similarity to the CGRP/migraine field that we hope to see in the AD field (!) is the arrival of oral small molecule treatments. Very soon after CGRP monoclonal antibodies were approved they were followed by small-molecule oral gepants.
 Key themes: biomarkers, particularly biofluid biomarkers, neuroinflammation, disease modification, and neuroplasticity
Key themes: biomarkers, particularly biofluid biomarkers, neuroinflammation, disease modification, and neuroplasticity
Jeffrey Cummings (Cleveland) showed the latest Phase I-III clinical trial landscape for Alzheimer’s disease and pulled out some interesting metrics. For example, whilst as expected the vast majority of the clinical pipeline is dominated by disease-modifying approaches (nearly 80%), most of these are small molecules (44%) rather than biologics (35%).

The neuroinflammation and synaptic plasticity assets in the clinical pipelines are interesting in that there is a wide diversity of targets being pursued, which kinda flies in the face of the herd-mentality criticisms we often hear about the industry. Don’t get me wrong, there is herd mentality, and there should be: I mean if you lost your keys in the house, the electricity is out, and it’s night-time, it’s no good going out to the street to look for your keys because of the street-lights – ‘There is more Light here’, so said the Mulla Nasrudin!
“Don’t get me wrong, there is herd mentality, and there should be…”
The diversity of targets being pursed in PI-III to modulate neuroinflammation (25) and synaptic plasticity (21)
From a ‘shots on goal’ perspective (what was that about hackneyed industry phrases?! ????), this is encouraging. However, the reason for this diversity of targets clearly reflects what we know: that biology is complex, often with built-in redundancy, and to thread the eye of the biology needle and find a drug with a good therapeutic index is tough. The long haul.
“Biology is complex, often with built-in redundancy, and to thread the eye of the biology needle and find a drug with a good therapeutic index is tough. The long haul.”
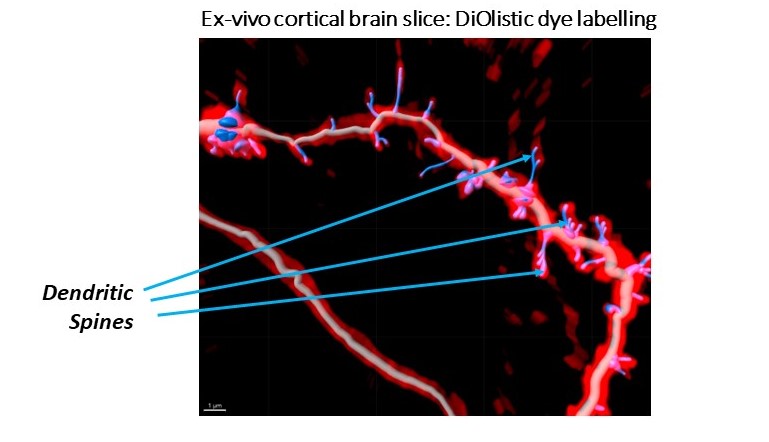
After thinking about the multiple targets being pursed to modulate neuroplasticity, which comes in various flavours, I was reminded of a conversation with an in-vitro biologist colleague (let’s call him Tim Philips, Associate Director, Bioscience). His team is trying to demonstrate neuroplastic changes in-vitro and ex-vivo (and considering immortalised cells v primary cells, vendor choice for cells, imaging platforms, neurite staining protocols etc). He juxtaposed the goal of in-vitro assays which is to generate as big an assay window as possible for screening purposes, and neuroplasticity assays where a few more neurites, spines, lengthened neurites, or slightly increased spine head widths might have real therapeutic benefit in diverse neurological or psychiatric diseases based on his reading of the literature. Is it enough? he asked. I don’t know. But working on targets ranging from SV2A, NMDA, muscarinic, sigma1/2, MAPK38, c-Met, PDE’s etc. is going to tell us more as these mechanisms are interrogated in neuroplasticity assays and tested in patients.
The exciting field of neuroinflammation
It is a field which has grown and grown now that we have thrown off the shackles of the ‘the brain is sacrosanct’ mantra. Well ok, it is, that’s why there is a blood-brain barrier which those developing small molecule drugs for CNS disorders know only too well. At this point, I will highlight you need a compound with an MPO score ideally ≥ 4 to show some chemistry credentials, but just wait for the next blog for something more detailed from our medicinal chemists….
…….but, back to Neuroinflammation
Think about all those targets that have been drugged or are being explored for disorders such as rheumatoid arthritis, psoriasis, cystic fibrosis, atopic dermatitis, and other inflammatory disorders, plus immuno-oncology approaches. Now pivot those to neurodegenerative disorders and you have a plethora of targets to attend to neuroinflammation, as illustrated at the 2023 AD/PD meeting. And you have more targets, such as human genetics (e.g, TREM2), the role of infections in neurodegeneration (e.g., periodontal pathogens), and targeting peripheral immune cells or peripheral anti- or pro-inflammatory pathways (e.g., PD-1) come to the fore, as we understand more about peripheral mechanisms influencing the CNS. Including peripheral immune cells infiltrating the CNS in AD and other neurodegenerative disorders (what was I saying about the brain being sacrosanct?).
So where do you start if you want to understand Neuroinflammation? – The LPS model of course!
Late last year I got downwind that a colleague with a huge knowledge of Immunology & Inflammation (let’s call him John Unitt, VP of Inflammation & Immunology) and his team were soon to start some systemic LPS challenge studies in rodents to measure peripheral cytokines. This team did not mess around, comparing the rodent’s effect of different LPS serotypes with full dose-response curves, measuring multiple cytokines in plasma, and looking at different time points over a series of studies.
I went straight to my beg, borrow, ‘steal’, and add-on mode. Enter stage left, Max asks: “Hey, can we get hold of the brains from those studies, just because?” “Sure”, John said. And it makes good sense from a 3Rs perspective too.
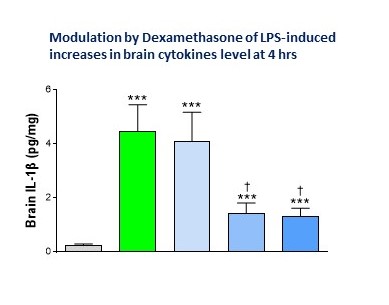
And so now we have measures of multiple cytokines in plasma and brain at different time points (plus qPCR brain cytokine measures) and are putting the brain tissue to good use to measure microglial and astrocyte activation (IBA1 and GFAP) and TSPO binding by autoradiography as a translatable biomarker of neuroinflammation.
“This team did not mess around, comparing in rodents the effect of different LPS serotypes with full dose-response curves, measured multiple cytokines in plasma, and looked at different time-points over a series of studies.”
I’ve rambled enough, but AD/PD 2023 was an excellent meeting and I’ll be going next year (Lisbon, is a wee bit warmer, methinks). A big thank you to the organisers……and a thank you to my Sygnature Discovery Inflammation & Immunology and Neuroscience colleagues for bolstering my personal knowledge in many ways.
Learn more about Max’s experience and work at Sygnature Discovery by visiting his bio.
