Cytotoxicity testing
Cell viability or cytotoxicity assessments are based on cellular functions that are particularly sensitive to toxic drugs, and as such provide convenient in vitro high-throughput screens (HTS) for comparing relative toxicities of a drug. And primary hepatocytes and/or hepatic cell lines are routinely used given that the liver is the target of most orally delivered drug toxicities.
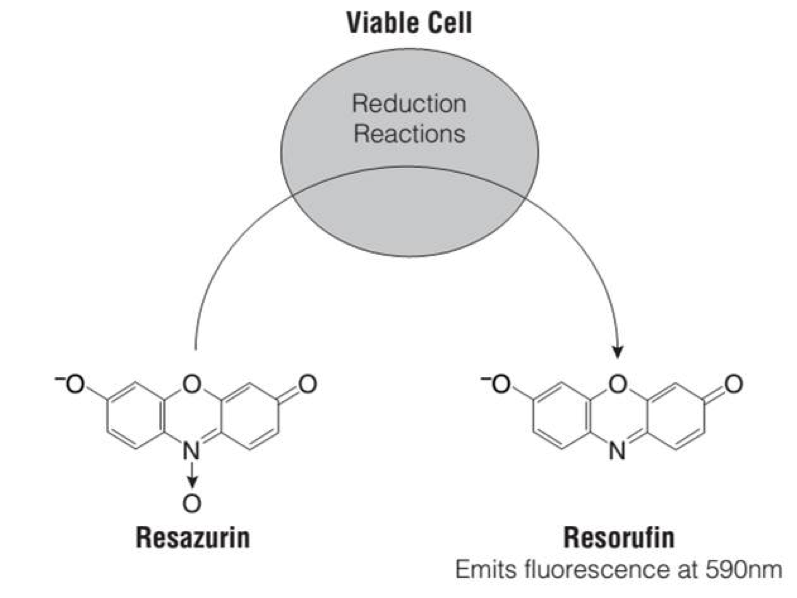
Sygnature’s Cell Viability assay is a homogenous method that uses the hepatoma cell line, HepG2 and uses a 96-well microtiter plate format to enable higher throughput screening of test compounds. The assessment involves determining the number of viable cells in culture, based upon the quantitation of an endpoint, namely resorufin (Figure 1), a pink fluorescent product of resazurin which signals the presence of metabolically active cells.
Briefly, the assay involves exposing live cells with functioning mitochondria to a set of test compounds and controls for a period of up to 24, 48 or 72 hours. At the end of this incubation 1 mM of Resazurin is added directly to cells cultured in serum-supplemented medium followed by a 4 hour incubation. The fluorescence of resorufin is then detected. Vehicle and digoxin are included as the negative and positive controls, respectively.
Typical pharmacokinetic profiles after a single intravenous (IV) or oral (PO) bolus.
