In Vitro to In Vivo Translation in Lead Optimization: Bridging the Biology Gap
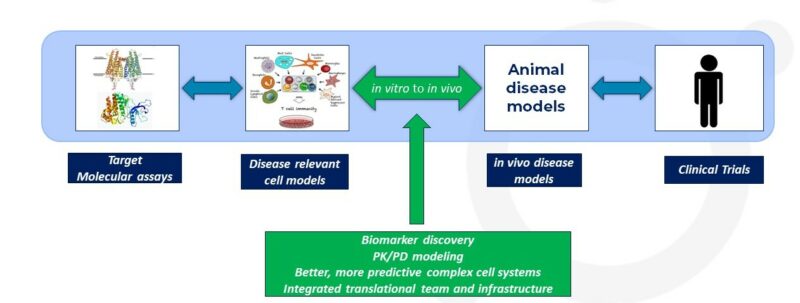
A generic in vitro/in vivo translational journey
Drug discovery is a complex and dynamic process that aims to identify and develop new therapeutic compounds to treat various diseases. One critical aspect of this journey is in vitro to in vivo translation: translating promising results from in vitro assays conducted in controlled laboratory environments to in vivo animal models and eventually to human clinical trials. This blog explores the significance of in vitro to in vivo translation in drug discovery, particularly the lead optimization phase, and highlights the challenges and strategies involved in this crucial process.
The Importance of In Vitro to In Vivo Translation
In vitro biological experiments play a fundamental role in the early stages of drug discovery. They involve screening potential drug candidates to support the DMT cycle using isolated cells, tissues, or biochemical assays to assess their activity, selectivity, species crossover, mechanism of action, in vitro ADME and safety. In vitro assays offer several advantages, including cost-effectiveness, reliability, high throughput and the ability to investigate specific molecular targets.
In lead optimization, the focus is on using these assays to drive the DMT cycle, to iron out any undesirable drug properties, like poor selectivity and ensure species cross over into the desired animal disease model and pave the way for successful testing in vivo. Equally important is discovering suitable biomarkers to measure the target engagement of the test drug in vivo and its concomitant efficacy on the disease. For example, when testing anti-inflammatory drugs in vivo, it’s common to measure specific blood cytokines as distal markers of target engagement and signs and symptoms (e.g., swelling, redness, body weight) as disease markers.
However, the ultimate goal of drug discovery is to develop safe and effective therapies for patients. A good drug translation to in vivo helps maximize and predict future project success in later-stage, expensive clinical trials. In vivo translation is crucial to determine how potential drug candidates behave within living organisms and disease models. In vivo studies provide valuable insights into drug pharmacokinetics (ADME), pharmacodynamics (PD: drug effects on the body), and overall efficacy in a complex (patho)physiological context.
For example, the acute LPS mouse inflammation model is ideal for testing the anti-inflammatory effect of a candidate drug on elevated blood and tissue pro-inflammatory cytokine levels to provide early insight into PK and PD. These in vivo studies are essential for assessing drug candidates’ safety, efficacy, and potential side effects before they can progress to clinical trials.
Learn more about lead optimisation in our latest eBook: ‘Lead optimisation: Delivering molecules fit for preclinical development’
Challenges in In vitro to In vivo Translation
Even with our current knowledge and expertise in translational sciences, the success rate of drugs is only 7% in drug development in 20221, mainly due to a lack of efficacy in the desired target patient groups.
- Biological Complexity: Living organisms are far more intricate than in vitro models, with a complex interplay between organs, tissues, and various physiological factors. Translating results from simplified static in vitro systems to the complexity of in vivo to dynamic models can be challenging and, under the influence of multiple factors, especially interspecies differences. A key challenge is accurately predicting the efficacy and safety of new drugs before clinical trials.
- Disease Model Validity: Selecting appropriate animal models or designing clinical trials that accurately reflect the disease pathology and treatment response in humans is crucial. The chosen models should possess sufficient similarity to human physiology and exhibit the desired disease characteristics and processes.
Strategies for Successful Translation
- Integration of In vitro and In vivo Data and Project Disciplines
Establishing a close scientific connection between in vitro and in vivo studies is vital. Bioscientists should design experiments with translation in mind, using in vitro models that represent the specific disease or target of interest accurately. The knowledge gained from in vitro studies should inform the design of subsequent in vivo experiments. Also, successfully bridging in vitro to in vivo translation is greatly helped by the close integration of drug discovery disciplines and team members, in particular, in vitro/in vivo pharmacologists and drug metabolism scientists. - Use of Predictive PK/PD Models:
Advanced computational modelling and simulation techniques can help predict human pharmacokinetics and the behaviour of drug candidates in vivo based on in vitro data. These models can aid in optimizing dosage regimens, predicting drug-drug interactions, and understanding potential side effects for human studies. - Use of More Predictive Animal Replacement In Vitro Models:
The ascent of more complex in vitro cell systems that more closely mimic the human disease in vivo is certainly gaining traction, even with the FDA2. 3D organoids, organs-on-a-chip, bioengineered human iPSC-derived cells, biosystem modelling and microfluidics systems with higher clinical disease biomimicry, all have contributed to predicting the human response to a new drug better than animal models. - Biomarkers and Surrogate Endpoints:
Identifying and utilizing reliable biomarkers or surrogate endpoints can facilitate the translation process. These indicators can help predict the efficacy and safety of drug candidates, providing valuable insights into treatment response and patient outcomes. - Collaboration and Data Sharing:
Sharing data, resources, and expertise can foster innovation and ensure the development of safe and effective drugs. Applying clinical learnings to lead optimization is important to improve the future clinical success of new drug discovery projects.
In vitro to in vivo translation is an integral part of the lead optimization process. It involves the translation of promising results obtained from a controlled laboratory in vitro experiments to more complex living organisms and, ultimately, to human clinical trials. Overcoming the challenges associated with this translation is essential to ensure the development and flow of safe and effective therapies for patients. Integrating in vitro and in vivo studies carefully, utilizing predictive PK/PD models, and fostering new, more predictive in vitro assays, will all help improve the success of this key process.
- Global Trends in R&D 2023. https://www.iqvia.com/insights/the-iqvia-institute/reports/global-trends-in-r-and-d-2023.
- FDA no longer needs to require animal tests before human drug trials. https://www.science.org/content/article/fda-no-longer-needs-require-animal-tests-human-drug-trials.
About the author
Dr John Unitt is Sygnature Discovery’s Vice President of Immunology and Inflammation Drug Discovery. John is a highly experienced pharmaceutical bioscientist and has worked in a broad range of drug discovery phases from biological target identification and validation, hit identification from multiple sources (e.g. HTS, natural products, fragment-based), novel assay development, hit to lead and lead optimisation.


