Do new cancer treatments mean cancer patients live longer?
In recent years, we have made great strides in cancer treatment. In study after study, the typical duration of progression-free survival (the time before the cancer starts to grow again) has increased significantly. However, in reality, the picture is considerably more complex. Sygnature Discovery’s Dr Allan Jordan explores.
When we take into account earlier diagnosis, and patients therefore knowingly living with their disease for longer, true survival for some types of cancer has barely changed over the past 40 years. [1] We are increasingly able to detect cancer earlier and earlier, but in reality, this simply leaves patients living under the cloud of cancer treatment for longer.
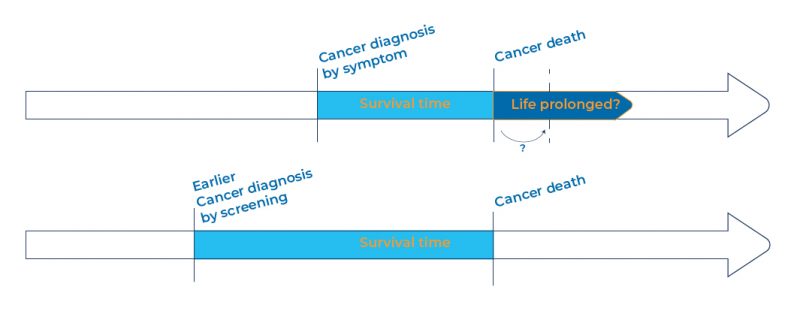
Given the decades of investment in cancer drug discovery, how can this be the case? Where we really seem to be struggling at the moment is to find cancer drug targets that actually eradicate the tumour. Even with earlier detection, by the time a patient hears they have cancer, many of their tumours are so disseminated and heterogeneous, with multiple sub-populations of cells, they are difficult to eradicate completely. If drugs are being used, it’s generally because the tumour has metastasised, and curative therapeutic options – such as surgery or radiotherapy – are no longer viable options.
We used to think that cancer was a very uniform, singular collection of cells. In reality, it’s not – it’s much more of a diverse community of different populations of cells. If we treat a patient with a particular drug, only a subset of that population of cells is likely to respond to treatment. Yet a tumour is likely to contain at least 108 cells before it can be detected via imaging. [2]
Statistical analysis suggests [3] that around 5000 of those cells will already be resistant to any individual drug. Resistance and recurrence are not simply unfortunate for the patients: it’s a mathematical certainty that a targeted cancer drug will fail in this setting. Once it has killed all susceptible cells, the resistant population will expand to become the dominant clone. This is, simply, evolution in an accelerated time-frame.
One way to circumvent this intrinsic resistance is to use two drugs in combination that work by different mechanisms, reducing the risk of leaving behind a resistant population of cells. Even with the blunt cell-killing tools of chemotherapy, combinations of drugs that act in different ways give a more durable response. Imagine what we could do with carefully chosen combinations of multiple, specific drugs. Recent clinical trial data suggest we are on the cusp of significant progress in this regard. [4]
Unfortunately, for many cancer types it is exceptionally rare that a cancer patient is cured by a drug. To overcome this challenge, we urgently need combinations of better drugs, better clinical trials, better cancer detection, and better genomic profiling so we can better match drugs to patients, delivering the right drug combination, to the right patient at the right time to give a response. If it were possible to dose combinations simultaneously at realistic therapeutic doses, the chances of the entire community of cancer cells in the body being eradicated would be correspondingly greater (and we’ll discuss that very topic in a forthcoming post).
At Sygnature, we have already progressed five potential oncology drugs into the clinic. We work closely with our clients to develop new drug molecules that we believe will work specifically and cleanly, and that can be combined with other drugs to significantly improve the outlook for cancer patients. We believe it’s time to reconsider what makes a good cancer drug for our patients. We believe it’s time to give our cancer patients more time.
We continually engage with our industry on a range of drug discovery topics. If you would like to discuss oncology drug discovery, our capabilities or even drug discovery in general then we’d love to hear from you. You can get in touch by using any of the contact forms.
[1] Cho et al, Journal of the National Cancer Institute Monographs, No. 49, 2014, 187-198
[2] Ugo Del Monte, Cell Cycle, 8, 2009, 505-506
