Bifunctional Degrader Drug Discovery: The Power of measuring EPSA
Targeted protein degradation has emerged as a powerful drug discovery tool for seemingly undruggable targets. These targets offer high potential for the development of new therapeutic agents. The pursuit of targets considered to be less druggable can result in drug discovery campaigns operating in “beyond rule of 5” (bRo5) chemical space. This category includes targeted protein degradation using bifunctional degraders, which connect a targeted protein binder and E3 ligase through a linker.
What is the problem?
In drug discovery, I believe that designing bifunctional degraders with acceptable oral pharmacokinetics still presents a significant challenge. These compounds have molecular weights exceeding 500 and can contain more than 5 H-bond donors and 10 H-bond acceptors. These molecular properties are required to deliver the essential compound interactions at both the protein of interest and the E3 ligase binding sites. This functionality results in these compounds having a much higher topological polar surface area (TPSA) than traditional small molecules. Yet, the high polar surface area can be detrimental to the ability of compounds to cross hydrophobic membranes. Absorption from the gastrointestinal tract can therefore be limited due to poor permeability. On the other hand, high levels of polarity are beneficial to compound properties such as aqueous solubility.
What features can make bifunctional degraders more permeable?
Compounds that are conformationally flexible can hide, or shield, some of their polarity, depending on their environment. These compounds can appear less polar when crossing a cell membrane. This property, known as molecular chameleonicity, is facilitated by the inclusion of multiple rotatable bonds in bifunctional degraders. These conformational changes can allow the formation of interactions such as intramolecular hydrogen bonds (IMHB). This can result in some of the polarity being buried when the compound experiences a hydrophobic environment. Similarly, the adoption of conformations that allow aryl or alkyl shielding of the polar groups depending on the environment can mask some of the polarity.1,2 The remaining exposed, or effective, polar surface area (EPSA) then determines the cellular permeability. Therefore, incorporating features in bifunctional degraders that reduce the EPSA can result in compounds with improved permeability.2
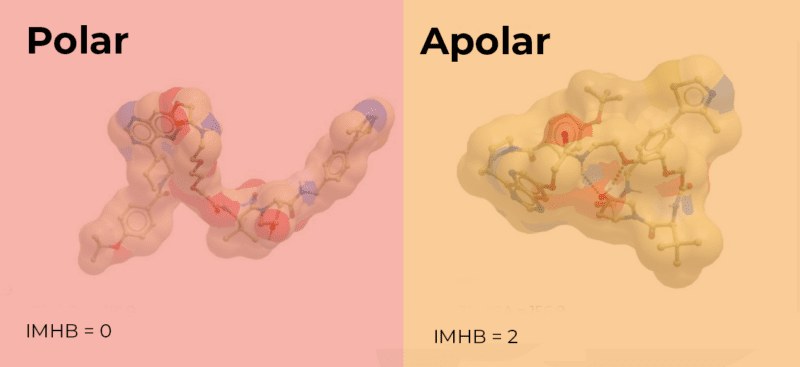
So, how can we assess the amount of exposed polarity?
Supercritical fluid chromatography employing a chiral stationary phase is used to measure the EPSA of bifunctional degraders.3 This technique provides a low dielectric constant environment, which is ideal for the formation of intermolecular hydrogen bonds with the compounds. Firstly, a set of calibration standards is used to establish a linear relationship between retention time and EPSA value. Subsequently, the retention time of test compounds is measured, and the EPSA values are calculated from the calibration line. To confirm compound identity, mass spectrometry is used in conjunction with chromatography. This technique has been used to measure the EPSA values of bRo5 compounds such as cyclic peptides and bifunctional degraders.2,4
Measuring EPSA values of bifunctional degraders
Sygnature Discovery’s CHARMED platform utilizes a high-throughput technique with low compound consumption, enabling rapid screening of multiple compounds. In my experience, this enables rapid measurement of EPSA values following the synthesis of a new compound and quickly assesses any modifications made to the molecule. Compounds with lower EPSA values have a greater chance of being cell permeable. By comparing the EPSA to the TPSA, we can assess how much of the polarity has been hidden. This assay forms a key part of the CHARMED platform.
What are the next steps?
By combining EPSA measurements with chromatographic LogD measurements, I believe that we can quickly characterise the physico-chemical properties of novel compounds, and identify the ones with the best chance of acceptable intestinal permeability. Once identified, these compounds can be prioritized for further profiling, including an assessment of their metabolic stability to avoid the impact of high levels of hepatic metabolism. Additionally, we can measure their solubility in biorelevant media to assess their absorption potential. Promising bifunctional degraders identified in these in vitro assays can then move on to in vivo PK studies to determine their oral bioavailability and potential as future human therapeutics.
- Sebastiano et al, J. Med. Chem., 2018, 61, 4189-4202.
- Caron et al, ACS Med. Chem. Lett., 2021, 12, 13-23.
- Goetz et al, J. Med. Chem., 2014, 57, 2920−2929.
- Ermondi, et al, ACS Med. Chem. Lett., 2021, 12, 1056-1060.
Dr Philip MacFaul is a Principal Scientist in the DMPK department at Sygnature Discovery.

