CellCentric & Sygnature Discovery: Success with Inobrodib – First-in-Class Oral Anti-Cancer Drug
Inobrodib, an exciting, new, first-in-class oral anti-cancer drug in clinical development by CellCentric, was collaboratively designed, synthesised and supported on its pre-clinical journey by an integrated project team at Sygnature Discovery.

The CellCentric and Sygnature Discovery management and project teams. Front Row Left: Simon Hirst, Barbara Young, Neil Pegg (Cellcentric CSO), Nigel Brooks (Cellcentric), David Laughton, Tomasz Knurowski (Cellcentric), Stuart Onions, Stuart Thomson, Jonathan Shannon, Gareth Harbottle
The drug targets the bromodomain of p300/CBP, epigenetic co-translational regulators of gene expression in the cell. The drug arose from a drug discovery programme which was initially targeting castrate resistant prostate cancer (CRPC), one of the most common cancers in men and a leading cause of male cancer deaths. Disruption of the bromodomain of p300 was shown to reduce expression of the androgen receptor and its variants which are important for driving late-stage disease (Figure 1).
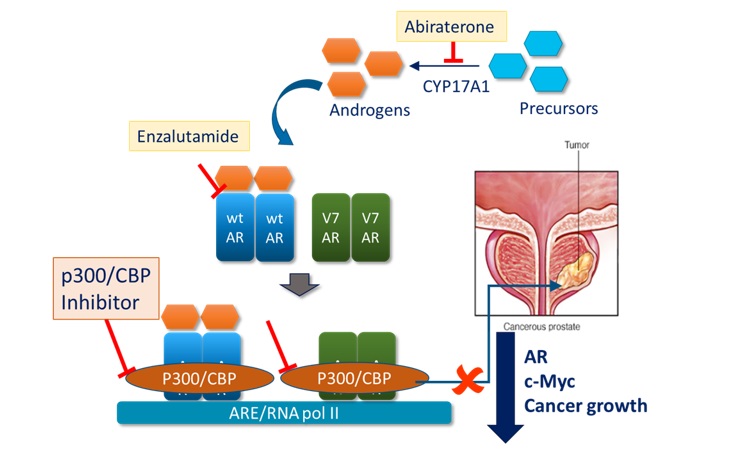
Figure 1: Mechanism of action of Inobrodib in CRPC
Working with CellCentric, our scientists demonstrated that the compound also disrupts expression of other specific cancer promoting genes, including MYC and IRF4 and this mechanism of action has enabled the drug to be trialled in other cancers where these co-translational regulators are important, such as blood cancers (multiple myeloma and acute myeloid leukaemia). It is also being targeted to other wide-ranging cancers which have loss of function mutations in either p300 or CBP such as lymphomas, lung, bladder and breast cancer.
Inobrodib was collaboratively designed and synthesised at Sygnature Discovery
Inobrodib, formerly known as CCS1477, was first designed and synthesised at Sygnature in June 2016 as part of a structure-based lead optimisation campaign to deliver potent, selective inhibitors of the bromodomains of p300/CBP. The integrated project team delivered innovative medicinal chemistry alongside a comprehensive, diverse screen cascade of in vitro assays covering potency, selectivity, mode-of-action, ex vivo biomarker, in vitro ADME and bioanalysis to deliver this drug. Using structure-based drug discovery and medicinal chemistry the joint CellCentric and Sygnature team improved potency whilst enhancing metabolic stability, selectivity and permeability. The chemistry was successfully scaled and optimised up to 200 g by the medicinal chemistry team to support preclinical studies. CCS1477 was made in 6 linear steps with an overall yield of 29% and chromatography was limited to two silica filter plugs.
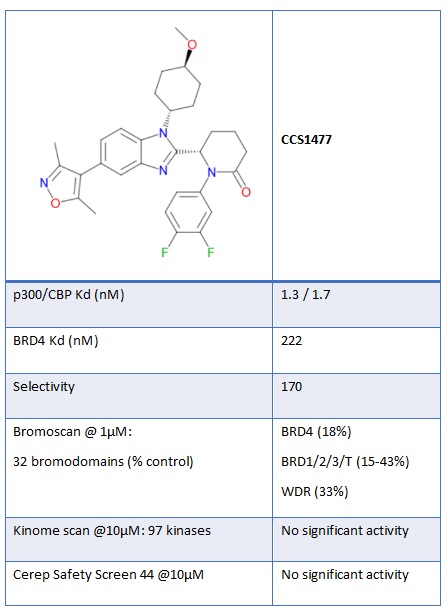
Figure 2: Chemical structure and in vitro potency of CCS1477
CCS1477, identified as a potent and selective small molecule inhibitor of the bromodomains of p300/CBP (Figure 2), is cell permeant with good in-cell target engagement, demonstrated through potent disruption of binding of the bromodomain of p300 to nuclear histones in a protein-protein interaction NanoBRET assay.
CRPC is associated with continued androgen receptor stimulation and gene expression changes through a variety of changes in the cell which drive the disease. CCS1477 was shown to have anti-proliferative effects against a range of prostate cancer cell lines and was able to reduce the expression of androgen-receptor and c-MYC regulated gene expression (Figure 3) suggesting it would have efficacy in this setting.
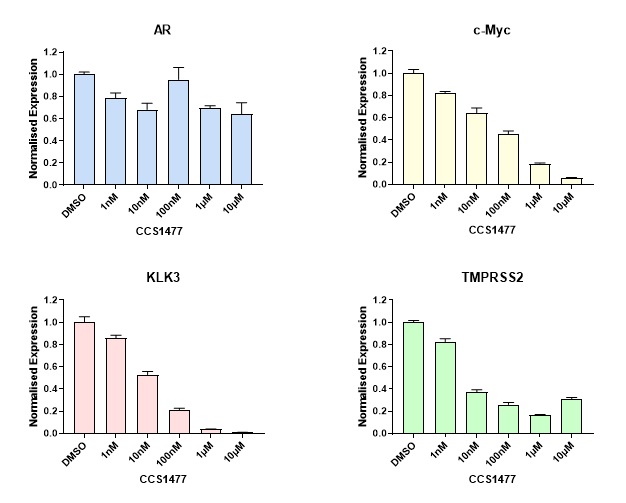
Figure 3: Inhibition of AR and c-Myc regulated gene expression by CCS1477
Inobrodib has good oral activity in appropriate in vivo models of disease
The drug is orally bioavailable, with rapid oral absorption, moderate clearance, and a low volume of distribution and in a 22Rv1 xenograft model of prostate cancer, CCS1477 administered at 10 mg or 20 mg/kg QD or 30 mg/kg QoD caused inhibition of tumour growth leading to tumour stasis over a 28-day treatment period (Figure 4, upper graph). When drug treatment was stopped, there was a prolonged period of sustained tumour growth inhibition until day 52 when the study finished. This study also indicated that it should be possible to dose intermittently and achieve good efficacy and thereby reduce any potential toxicology issues. The unbound drug exposure causing stasis in the 22Rv1 mouse xenograft model was used to derive a target human exposure.
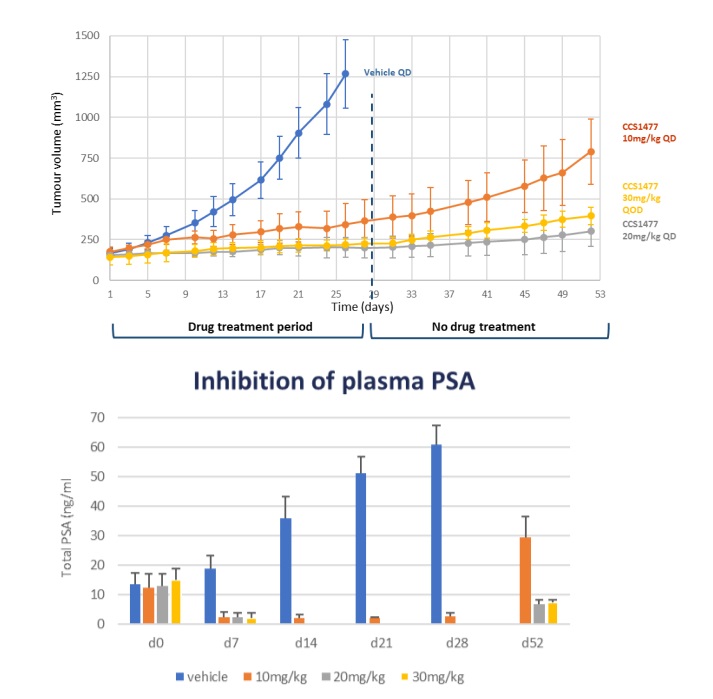
Figure 4: In vivo activity of CCS14877 in 22Rv1 xenograft model showing clear inhibition of tumour growth inhibition which is sustained on drug withdrawal (upper figure) and associated with a reduction in plasma PSA (lower figure).
Treatment with CCS1477 was associated with highly significant inhibition of plasma PSA, a clinically relevant biomarker (Figure 4), as well as tumour AR-FL, AR-SV and c-Myc protein and KLK3 and TMPRSS2 genes in this model, demonstrating good linkage between in vitro and in vivo biomarker results and providing appropriate biomarkers for use in the clinic.
In a similar manner, the project team were able to demonstrate CCS1477 was able to inhibit proliferation of haematological cancer cell lines, impacting cMYC and IRF4 regulated gene expression, enabling inobrodib to be trialled in a wider range of cancers where p300 and/or CBP may have a role in driving the disease.
These exciting findings were presented by CellCentric as a ‘New Drug On The Horizon’ at AACR in 2019 and as a pivotal publication in Cancer Research in May 2021, with many Sygnature co-authors.
Findings in animal models also helped with dose to man predictions
Findings in animal models also helped the team at Xenogesis (now part of the Sygnature Discovery) to predict the appropriate dose for this oral anti-cancer drug in the clinical trials. The project was supported using a physiologically based pharmacokinetic (PBPK) modelling approach with clearance in mouse and dog predicted within two-fold and rat within three-fold from in vitro data. Volume of distribution was predicted within two-fold for all three species. Using human in vitro hepatocyte and plasma protein binding data, the predicted clearance in man was low with a moderate volume of distribution. Based on matching unbound exposure in pre-clinical tumour growth inhibition studies, efficacy in man was predicted to be achievable with an acceptable dose regime.
“This was a first class collaboration and we couldn’t have suceeded without Sygnature Discovery’s efforts“
– Neil Pegg, CSO, CellCentric
Inobrodib is currently in Phase1/2a dose escalation and dose expansion study trials. A good therapeutic window has been demonstrated with this oral anti-cancer drug, with clear anti-tumour effects in late stage CRPC patients as a monotherapy, and in combination with standard of care agents. Initial clinical trials in blood cancers have also shown clear results and is continuing to progress both as monotherapy and in combination with existing agents. Clearly inobrodib, a drug first identified at Sygnature for CellCentric, is a success story and this drug is already making a difference to patients. It has the potential to transform the outcomes and quality of life for people with a range of specific cancer types.

Neil Pegg, CSO, CellCentric and Simon Hirst, CEO, Sygnature Discovery
About Sygnature Discovery
Sygnature Discovery is a leading independent integrated drug discovery company. The team provides drug discovery services to find novel ways to fight incredibly serious diseases. Private equity-backed by Five Arrows Principal Investments, we operate fully-enabled research facilities in Nottingham and Alderley Park, UK, which house over 400 research scientists (over 80% of whom hold a PhD). Additional offices are located in Cambridge, MA, and South San Francisco, CA, in the US.
Since 2011, thirty-six compounds, discovered by Sygnature for partners, have entered pre-clinical development. Twenty-one of these have progressed to clinical trials (Phases I, II and III).
Read another success story from Sygnature Discovery:
